The Military Health Systems Research Symposium (MHSRS) hosted at the Gaylord Palms Resort in Kimmissee, Fl on Aug 14 thru 17, 2023. MTEC was fortunate to have a booth large enough to host a small poster session as an extension of the larger MHSRS poster session. Over the course of three days, we rotated through 36 different MTEC members presenting the latest data and advancements for their products. We appreciate all of the presenters taking the time to walk us through their exciting product developments. Several of these presentations can be found below, divided into the following sections:
Section 3: Human Performance and Regenerative Medicines
Section 4: Regenerative Medicines
Section 5: Infectious Diseases
Section 6: Simulation and Training
Section 7: Other Casualty Care
We are grateful for all of the MTEC members that participated at MHSRS. We look forward to seeing you all again next year!
Section 1: Brain Health
Section 1: Brain Health
“Handheld Brain Diagnostics”
Infrascanner is the only handheld device in service that can detect brain hemorrhage at near 95% sensitivity and specificity levels. Partnering with USAMMDA and MTEC, Infrascan is developing the next generation of this technology to be available for use by the Marine Corps and all services, as well as funding research toward potential detection of brain hemorrhage expansion.
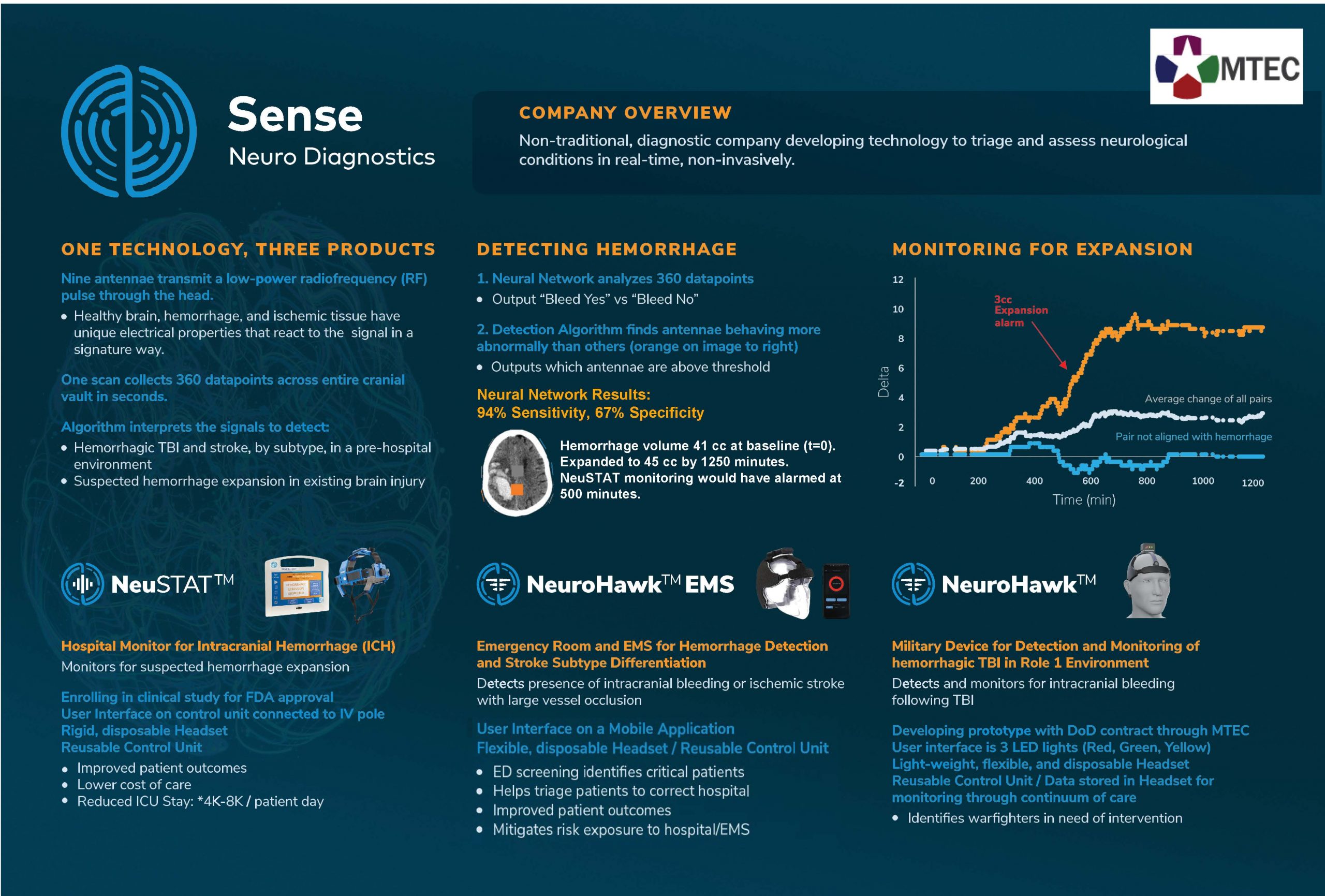
“Overview of the Sense Technology”
Sense Diagnostics is developing three devices using the same core technology to detect and monitor hemorrhage in stroke and TBI subjects.
New Jersey Institute of Technology
“Engineering Pre-Clinical Models for Traumatic Brain Injury Research”
Integrating engineering, biology and medicine to address Traumatic Brain Injury (TBI) through experimental, computational and clinical methods.
“Vista LifeSciences Health Technologies”
Vista LifeSciences (VLS) is a healthcare technology company that provides cognitive and behavioral health assessments, mobile health records, and data management and analytics solutions for industry, military, and community.
Section 2: Hemorrhage, Blood
Section 2: Hemorrhage, Blood
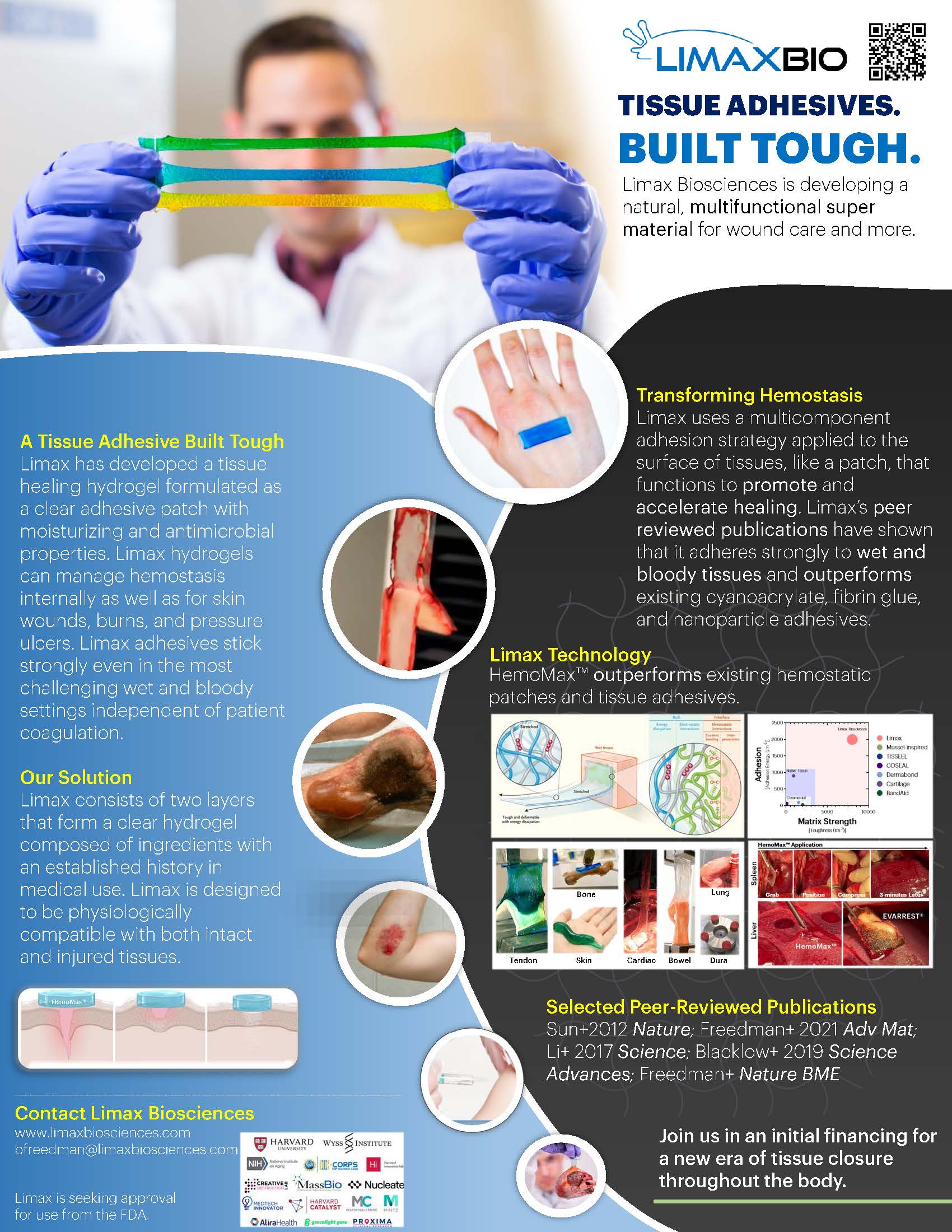
“Tissue Adhesives. Built Tough.”
Limax Biosciences has developed a next generation, stretchable hydrogel-based tissue adhesive to revolutionize treatments for injuries inside and outside the body. Existing hemostatic dressings and sealants have weak tissue adhesion that result in leaks, tissue rigidity, and cytotoxicity. Limax’s degradable, hydrogel-based material addresses these limitations through strong and rapid adhesion to wet tissue surfaces at 10-100x higher the strength of competitors. This patent-protected platform technology can be used in several indications throughout the body including soft tissue reinforcement and local drug delivery.
“Platelet-Inspired Therapeutics for Hemorrhage Control and Blood-Related Disorders”
Haima Therapeutics is a pre-clinical stage biopharmaceutical company developing a platelet-inspired drug to mitigate bleeding
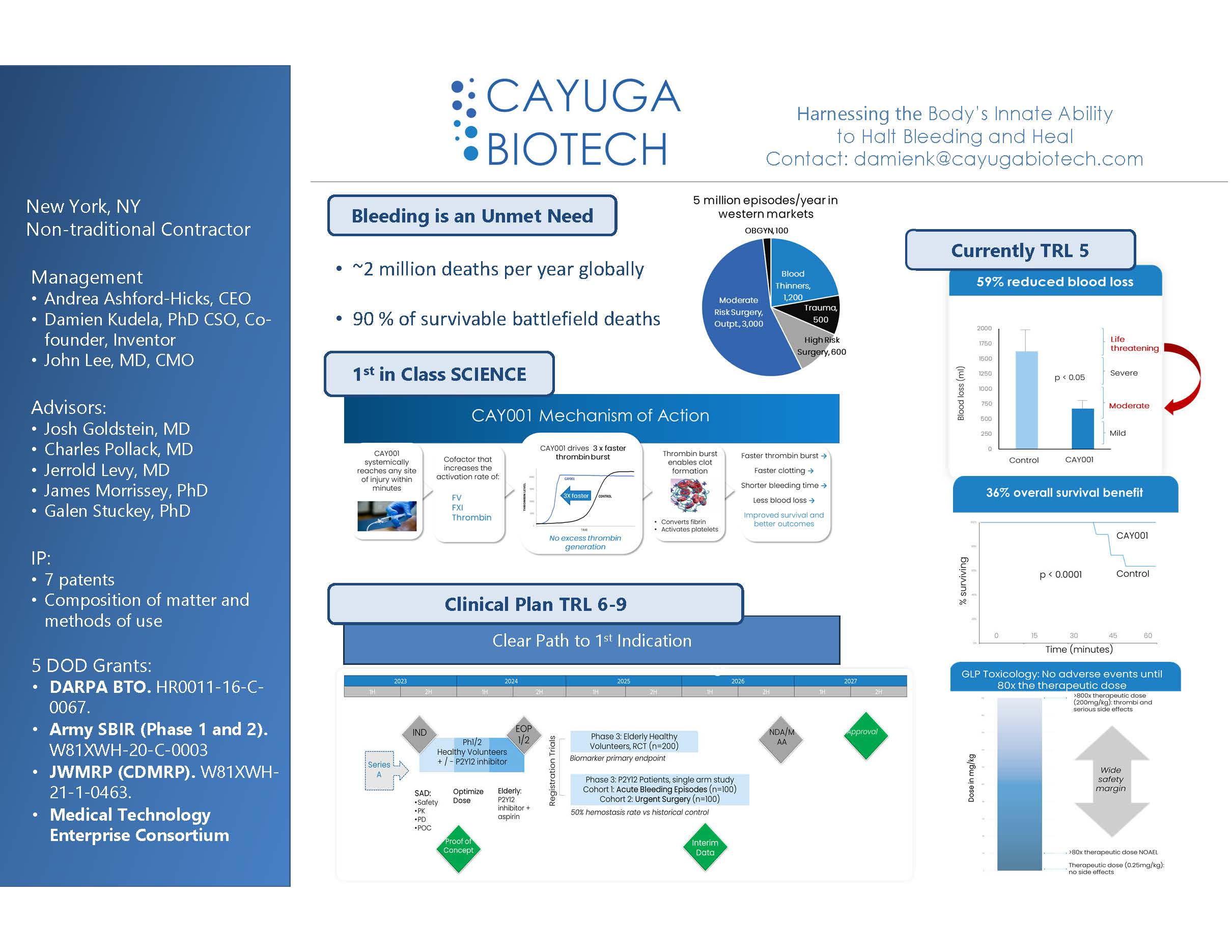
“CAY001 – Harnessing the Body’s Innate Ability to Halt Bleeding”
CAY001 is an IND-ready, intravenous therapeutic designed to treat severe bleeding episodes for both military and civilian use even in austere conditions.
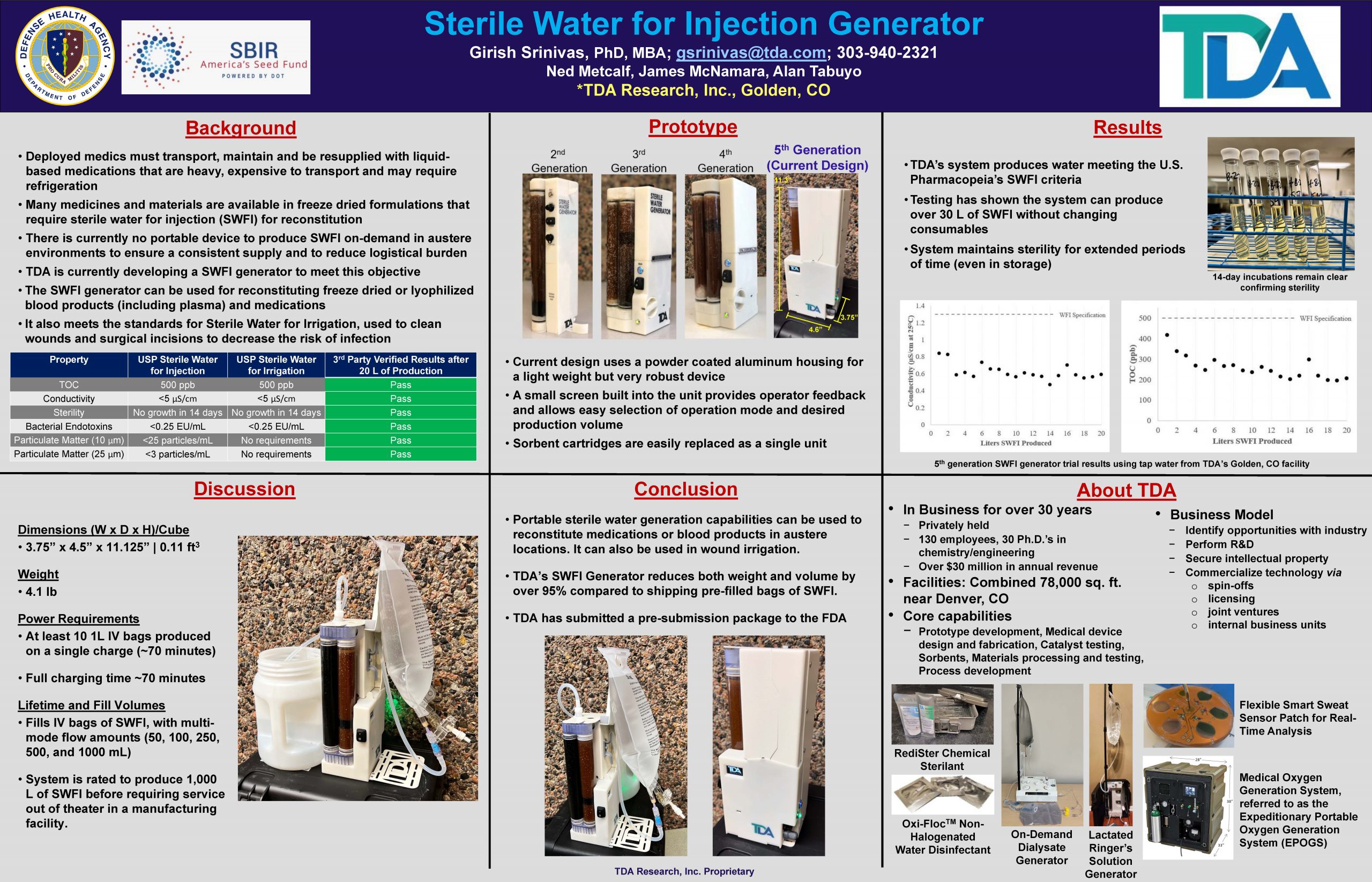
“Sterile Water for Injection Generator”
A man portable and ruggedized sterile water for injection (SWFI) generator to produce SWFI from potable water in-theatre for rehydration of drug and blood products, or sterile water for irrigation for cleaning wounds and surgical incisions.
Section 3: Human Performance
Section 3: Human Performance
“HsPro® – Transition into Production of an Innovative Multi-Parameter Wearable Sensing Platform for Physiological Status Monitoring (PSM) in Highly Dynamic Aviation Scenarios”
The objective of the HsPro® program was to transition previously completed USN RDTE on an aviator sensing platform (HAMS I, HAMS 2, and HMAPS) into production while improving performance in four areas: improved wearable comfort for users, improvement in near real time signal acquisition, improved battery life with both BLE and Wi-Fi connectivity (dual radio), and physiologic sign monitoring design validation.
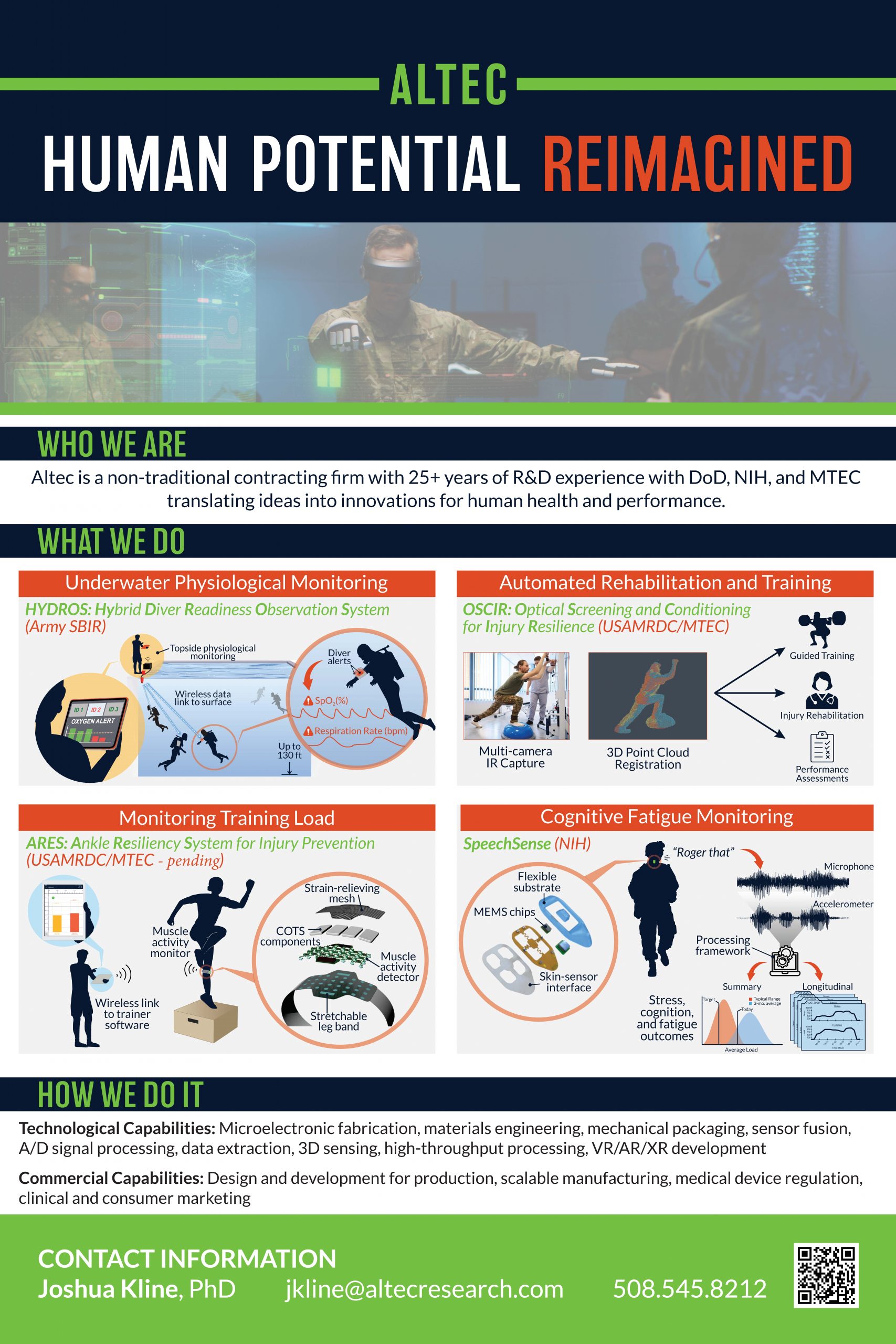
“Technologies for human health and performance”
Altec, works to develop innovative technologies to solve our nation’s toughest challenges in health and human performance.
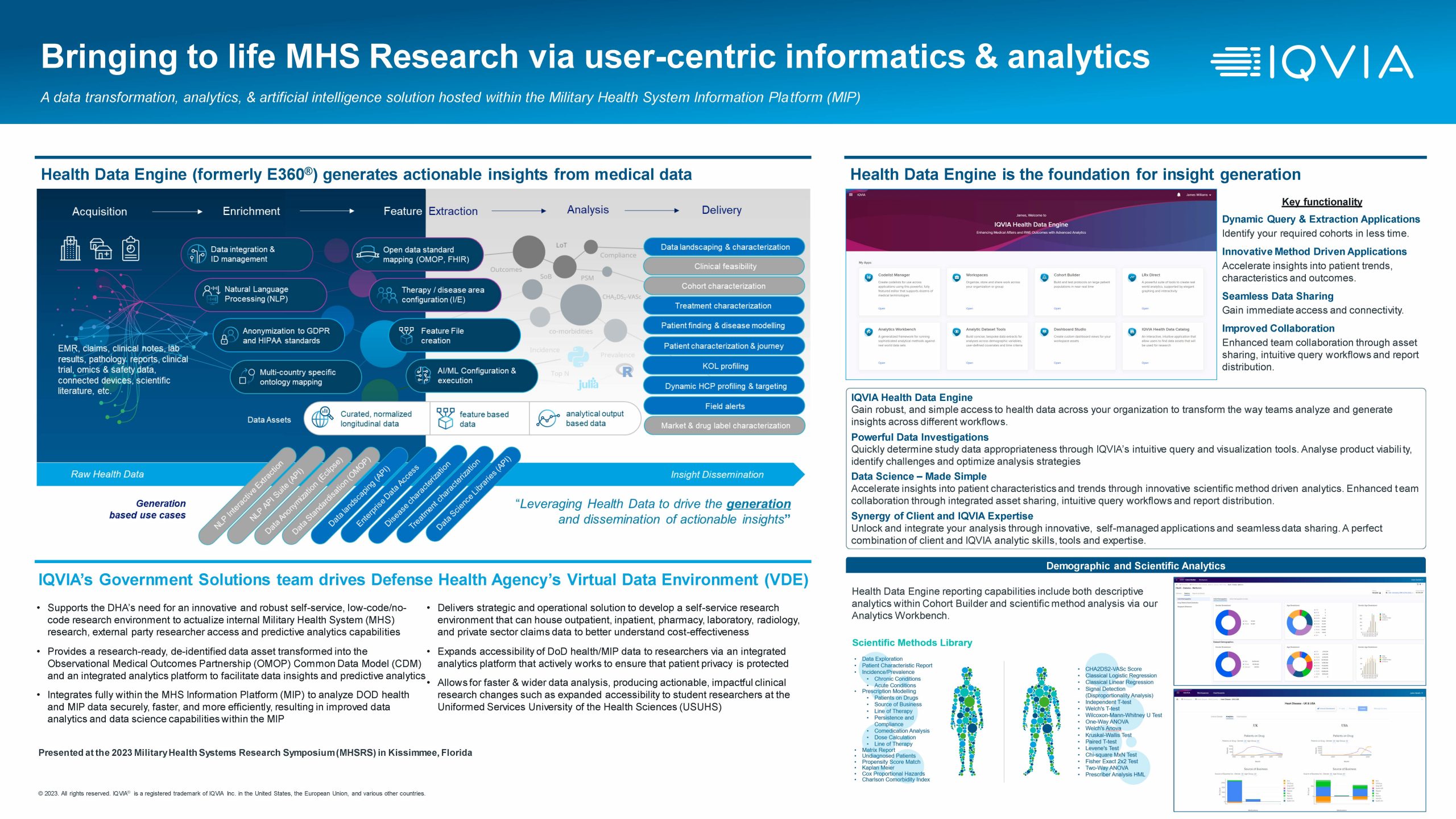
“Bringing to life MHS Research via user-centric informatics & analytics”
IQVIA fully integrates a research-ready, de-identified data asset transformed into the Observational Medical Outcomes Partnership (OMOP) Common Data Model (CDM) and an innovative and robust self-service, low-code/no-code research environment within the MHS Information Platform (MIP) to analyze DOD health and MIP data securely, quickly, and efficiently.
“Technology Partner for Movement Health Innovation”
Sparta’s Movement Health Platform (MHP) provides secure data collection, management, analysis, and decision support capabilities enabling organizations to establish the foundation for scalable, data-driven, Human Performance optimization research and operational decision support.
Section 4: Regenerative Medicines
Section 4: Regenerative Medicines
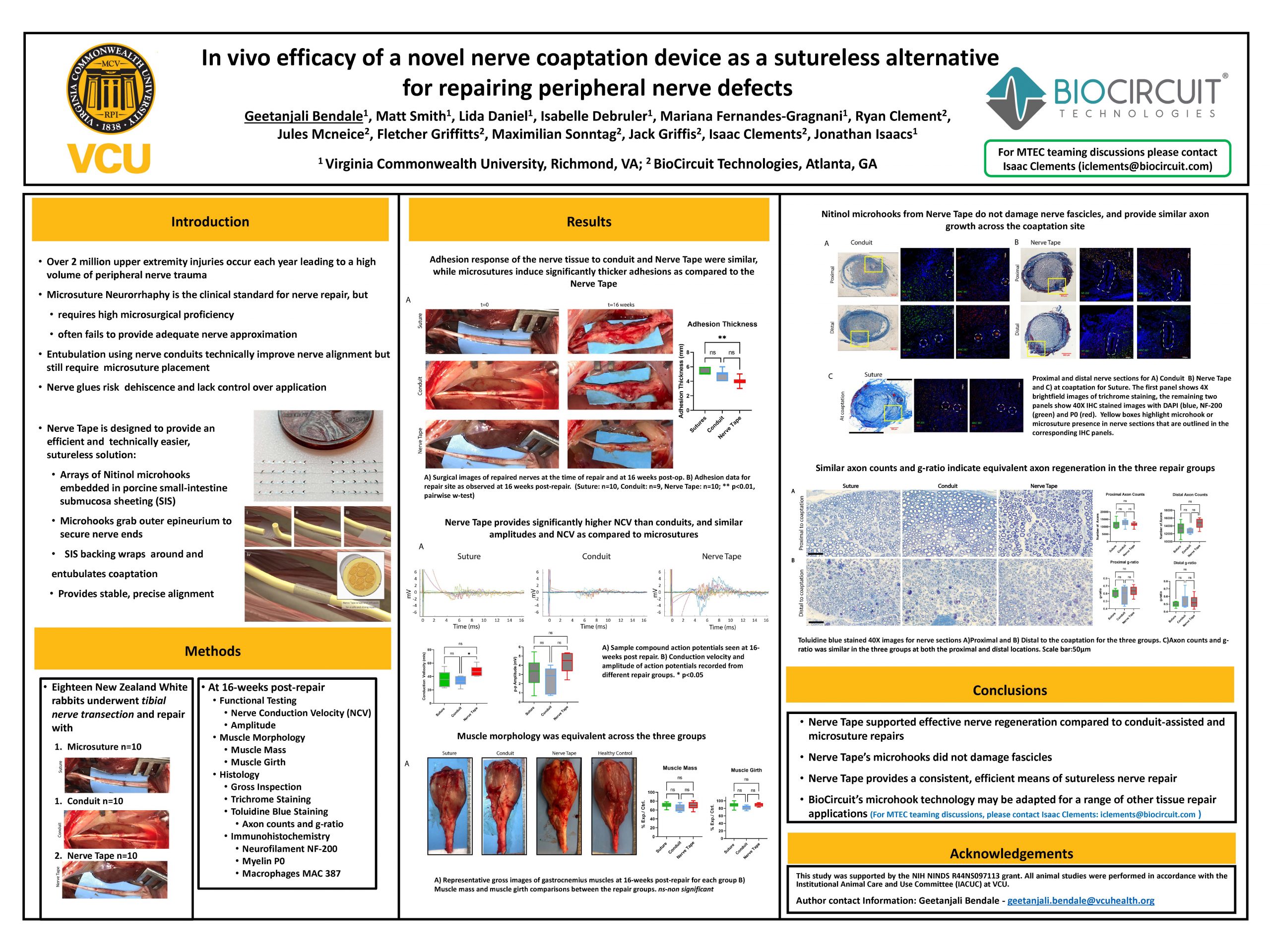
“In vivo efficacy of a novel nerve coaptation device as a sutureless alternative for repairing peripheral nerve defects”
Nerve Tape enables rapid, higher quality nerve repairs, without the need for suturing.
“Intelligent Factory in a Box Vision”
Production, Scale up, and Foundation for Future Distributed Medical Manufacturing Innovations. Moving from a manual process to a fully automated and validated manufacturing for biocompatible and implantable medical devices.
Section 4: Infectious Diseases
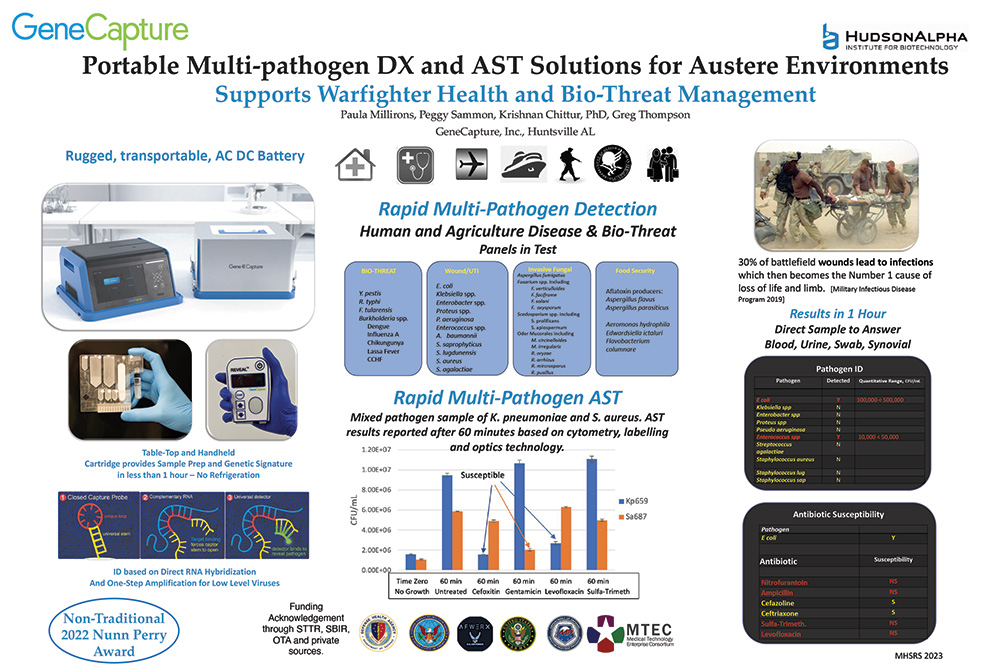
“Portable Multi-pathogen DX and AST Solutions for Austere Environments”
GeneCapture’s product development now includes operating prototypes for remote rapid multi-pathogen detection and antibiotic susceptibility testing.
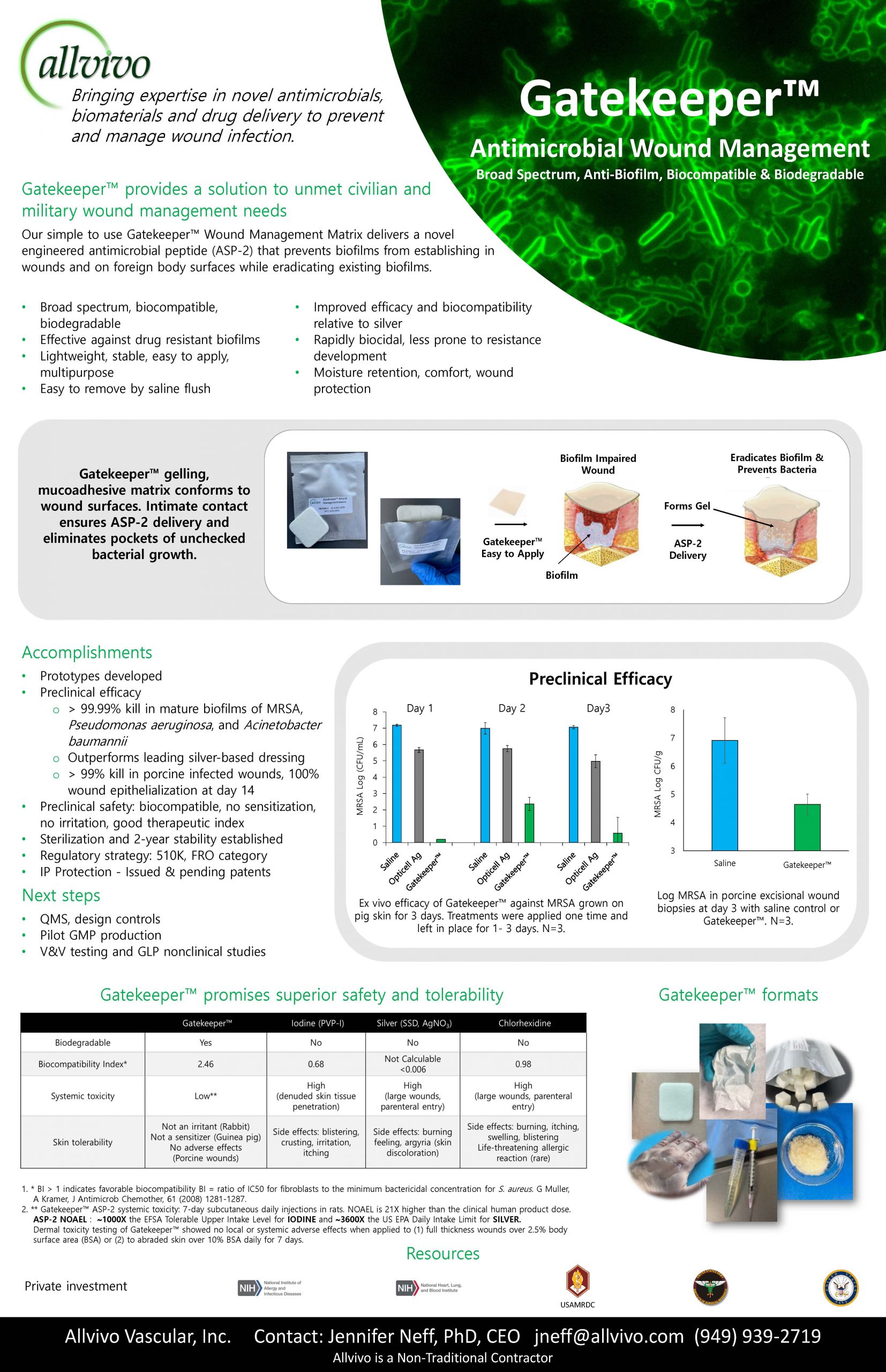
“Gatekeeper™ provides a solution to unmet civilian and military wound management needs.”
Allvivo’s Gatekeeper Antimicrobial Wound Management Matrix provides a new approach to prevent and manage wound infection using a broad spectrum antimicrobial peptide that is effective against multidrug resistant bacteria and their biofilms.
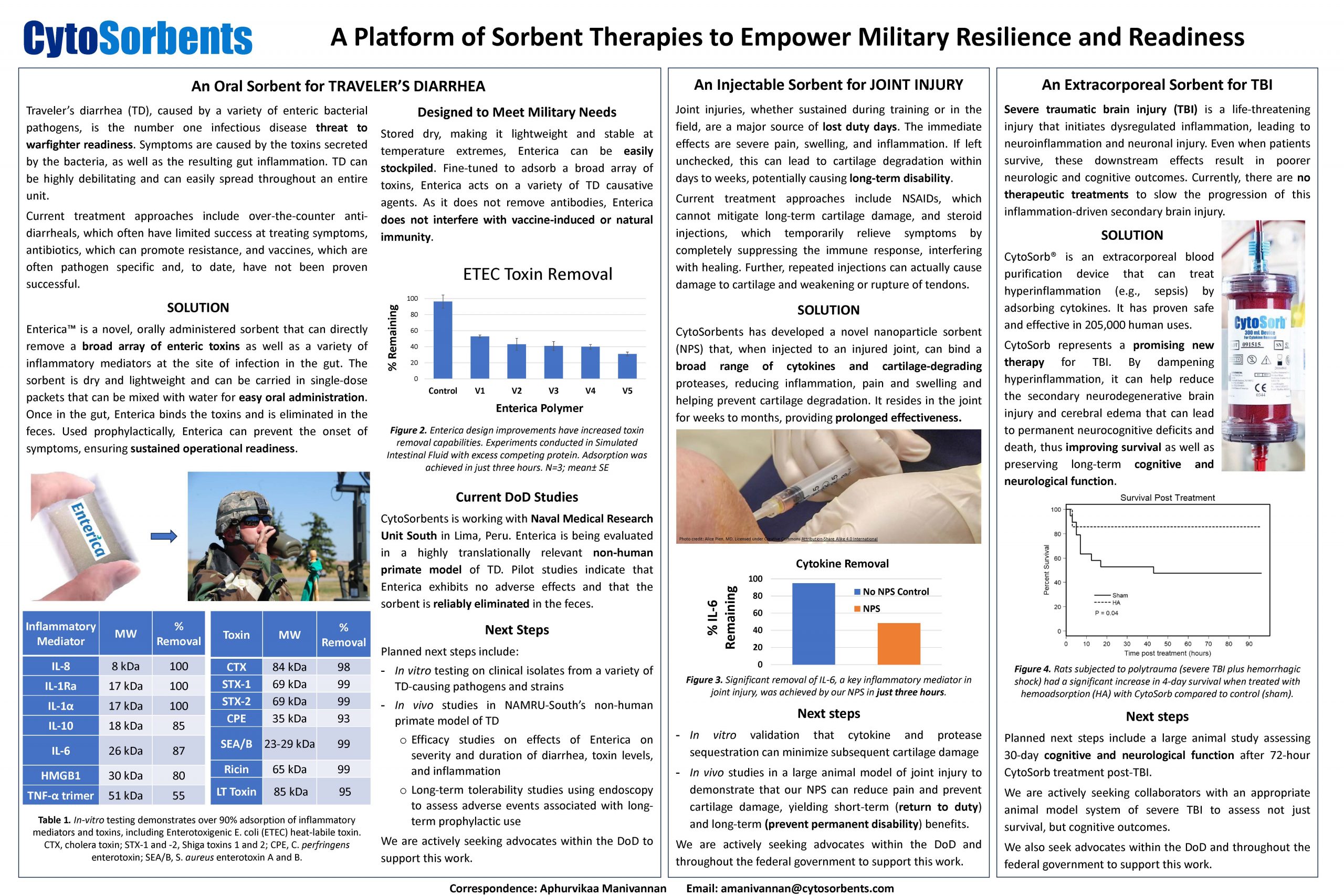
“A Platform of Sorbent Therapies to Empower Military Resilience and Readiness”
CytoSorbents looks to address some of the military’s most impactful health issues, helping prevent illness, speed recovery, and improve outcomes. We do this with a portfolio of sorbent-based treatments designed to remove harmful substances from the body. Our products in development include an oral sorbent to prevent pathogen induced diarrhea, an injectable sorbent that can speed recovery from joint injury, a blood purification device that can improve survival and preserve cognition after traumatic brain injury.
“Biologics License Application (BLA) of a Bovine Immunoglobulin Supplement that prevents Travelers’Diarrhea caused by Enterotoxigenic Escherichia Coli (ETEC).”
Immuron is pursuing a regulatory pathway to license Travelan, a commercially available oral prophylactic hyperimmune bovine colostrum (HBC) immunoglobulin product with the Food and Drug Administration (FDA) via a Biologics License Application (BLA), with a proposed indication to prevent Travelers’ Diarrhea.
Section 6: Simulation and Training
“Synthetic Training Environment for Multimodal Medical Training (STEM3T)”
With support from the Medical Simulation & Information Sciences Research Program (MSIS) at the U.S. Army Medical Research & Development Command (USAMRDC), Vcom3D is developing scenario generation tools, assessment instruments, and learning system interfaces that enable the development of multi-modal (live, virtual, manikin) medical training scenarios using the Modular Healthcare Simulation and Education System (MoHSES) standards. These have the potential to lower cost and increase interoperability of medical simulation systems for multi-modal training that can be used by all services at multiple echelons of care.
Section 7: Other Casualty Care
Berthiaume Institute for Precision Health – University of Notre Dame
“Wound Healing Promoted by Broad-Spectrum, Phage Structure Mimicking, Synthetic Antibacterial Nanoparticles as a Topical or an Intravenous Formulation, which Successfully Treated MDR ESKAPE Pathogens Induced Infections in Wound Models”
Intravenous or topical, broad-spectrum, Phage-Mimicking, antibacterial nanoparticles with a bactericidal action against the ESKAPE pathogens, without the emergence of resistance, as a viable alternative to antibiotics formulations.
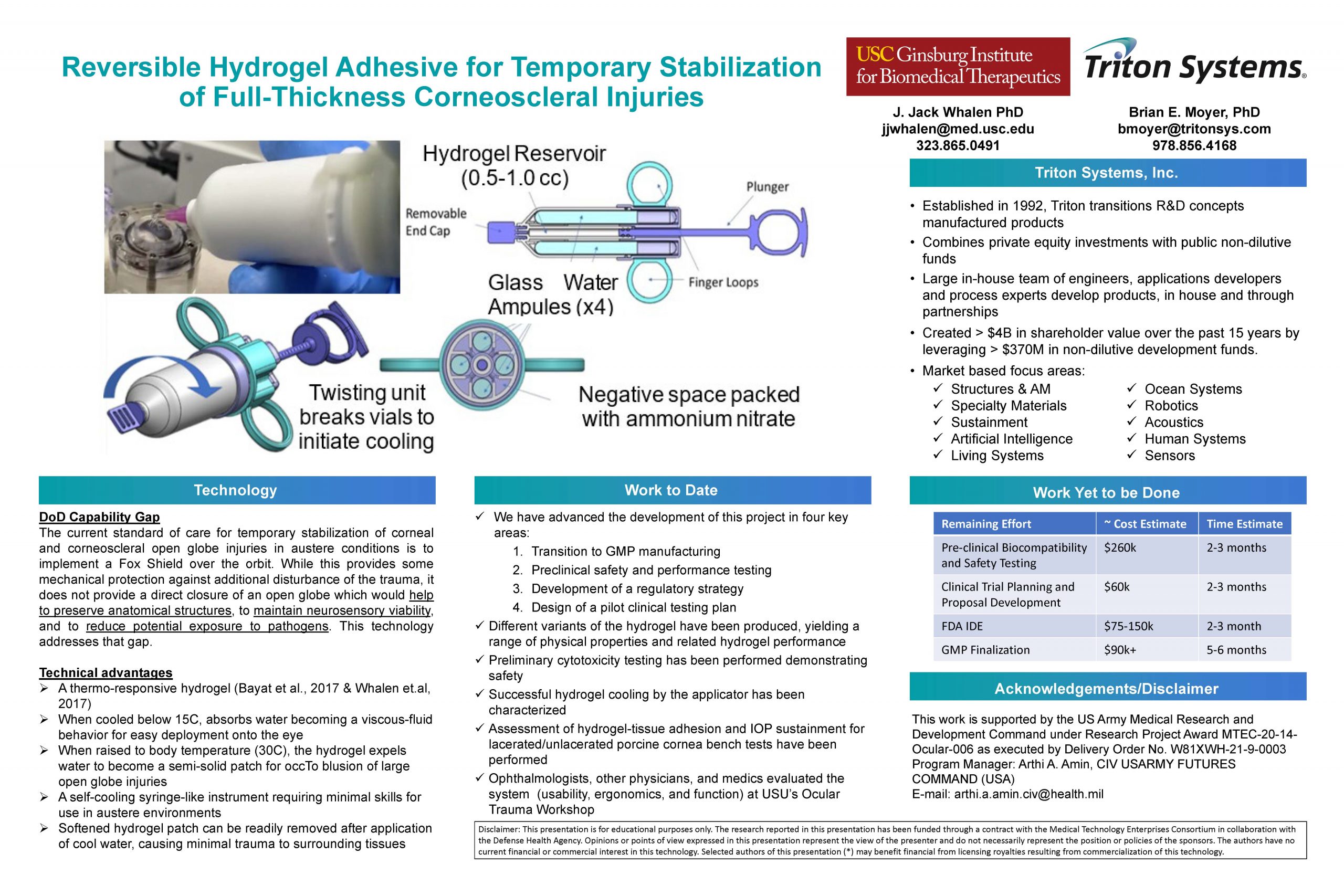
“Reversible Hydrogel Adhesive for Temporary Stabilization of Full-Thickness Corneoscleral Injuries”
This poster summarizes ocular trauma occlusion device development effort, based on a reversible hydrogel, that has been performed to date and work remaining to support an FDA IDE submission.
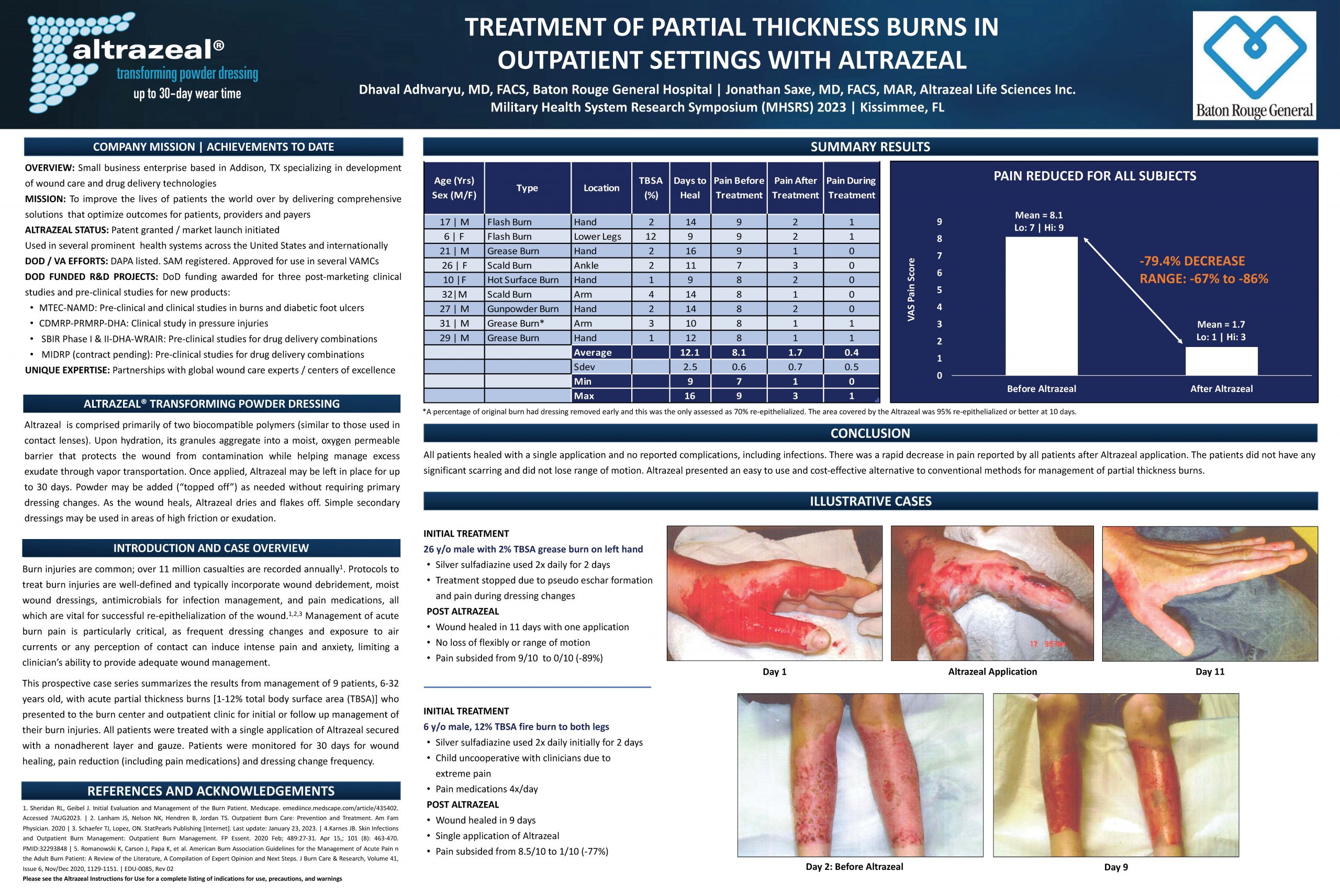
“Treatment of Partial Thickness Burn Wounds in Outpatient Settings with Altrazeal”
A 9-patient prospective case series evaluating the efficacy of Altrazeal in treating partial thickness burn wounds in outpatient settings, demonstrating its potential as a versatile and convenient wound management option
“AI-supported Sonographic Detection of Intracranial Hypertension and Other Trauma-related Tasks”
AI-supported ultrasound technology, developed by Kitware, can reduce the operator training burden and allow for in-field detection of intracranial hypertension (e.g., in cases of severe traumatic brain injury) and other trauma-related tasks (e.g., detection of pneumothorax, nerve block placement).
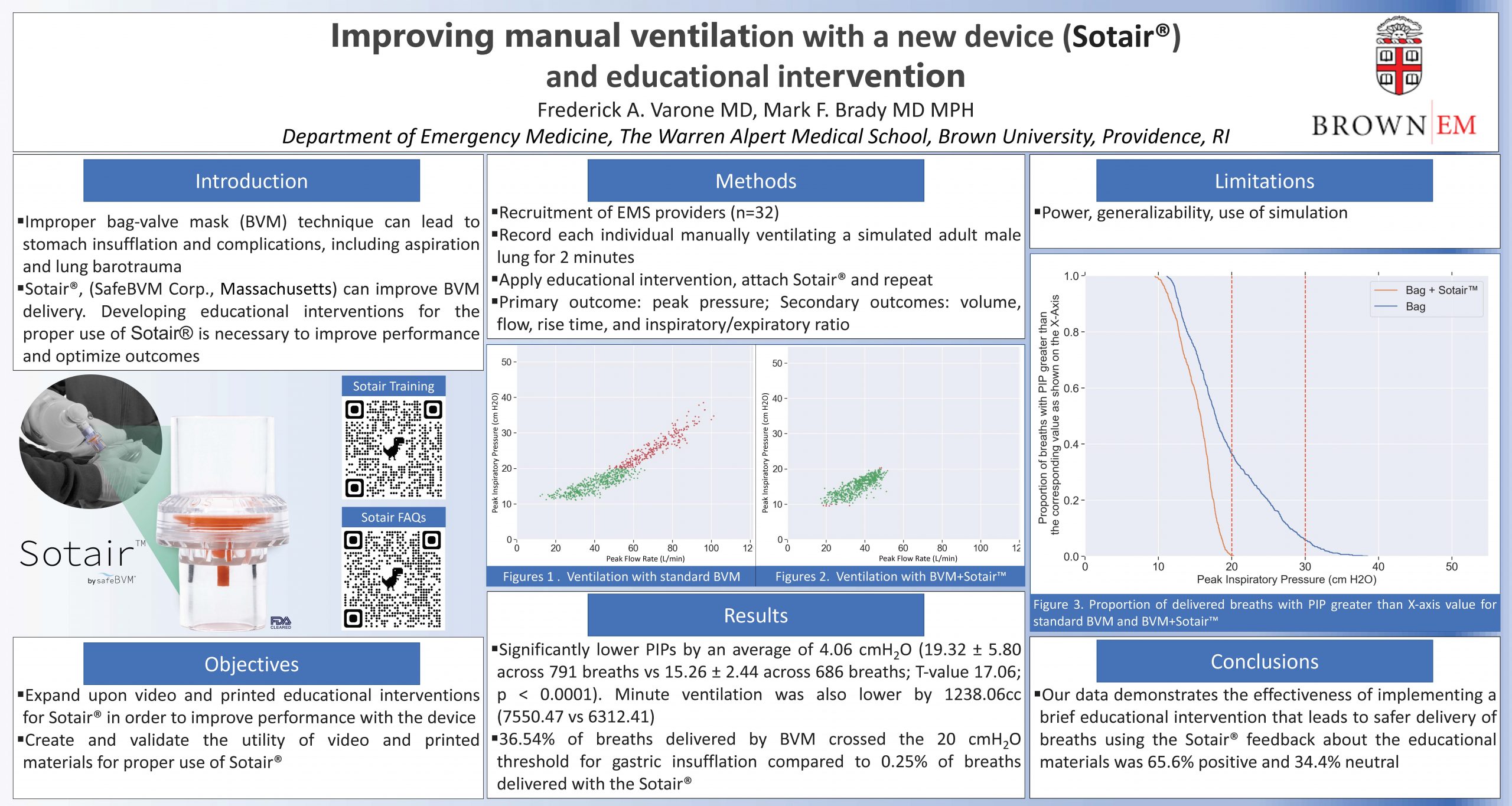
“Improving manual ventilation with a new device (Sotair®) and educational intervention “
One sentence description of the poster: Improper bag-valve mask (BVM) technique can lead to stomach insufflation and complications, including aspiration and lung barotrauma, Sotair® (SafeBVM Corp) and brief education can improve BVM delivery.
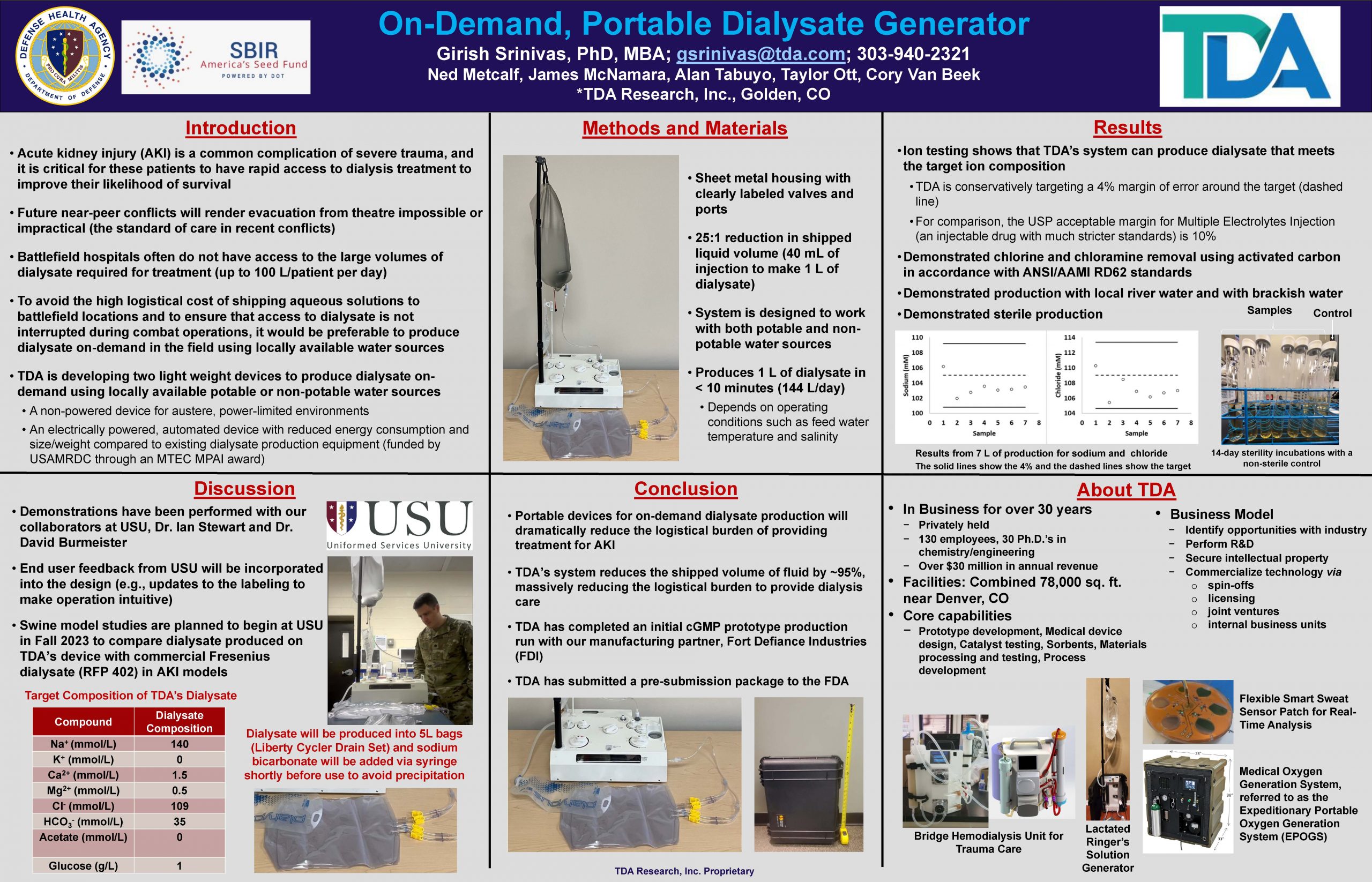
“On-Demand, Portable Dialysate Generator”
A man portable, ruggedized, and non-powered dialysate generator to produce dialysate on-site with any available fresh water for in-theatre dialysis treatment.
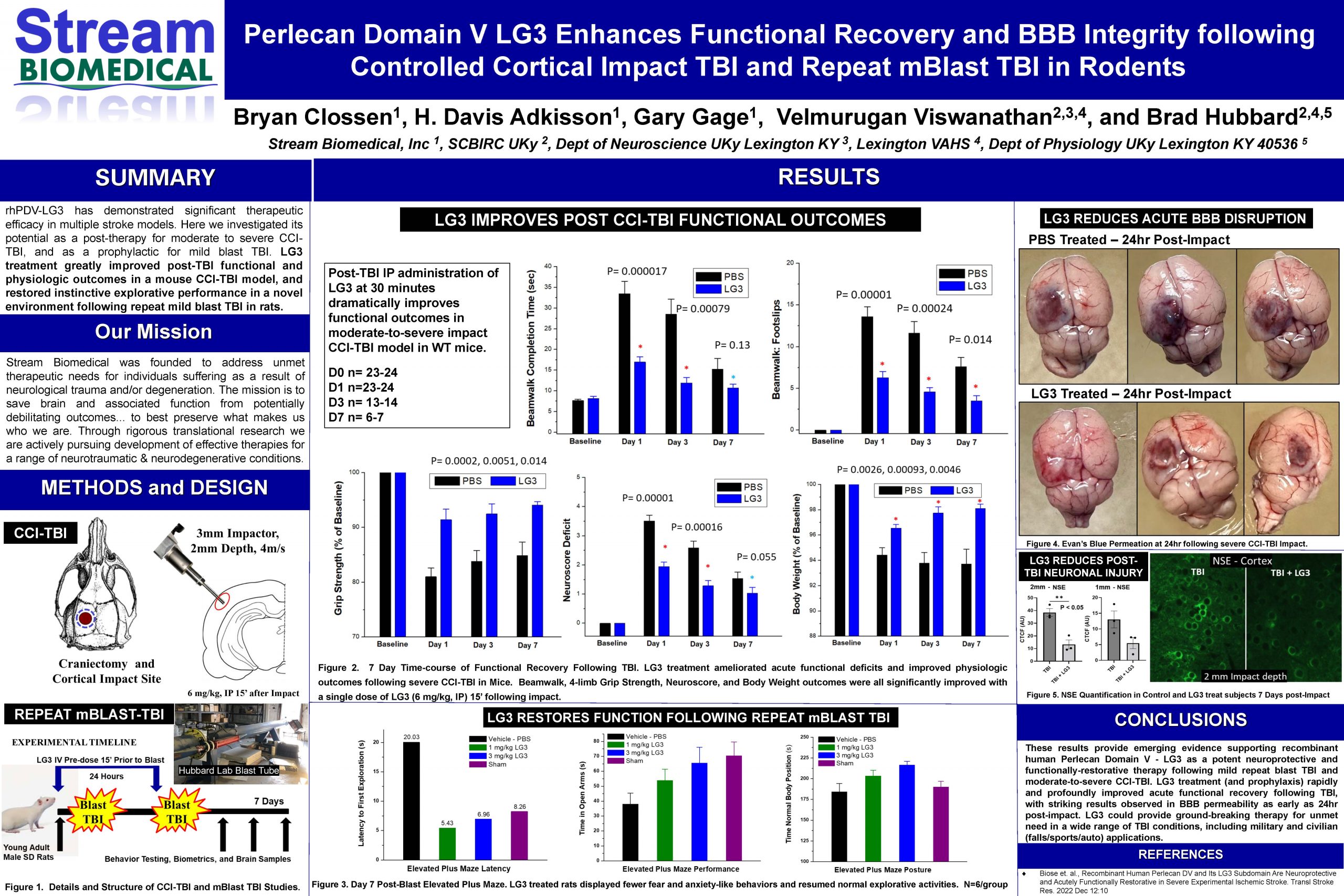
“Perlecan Domain V LG3 Enhances Functional Recovery and BBB Integrity following Moderate-to-Severe Controlled Cortical Impact TBI and Repeat Mild Blast TBI in Rodents”