

MTEC GROWTH DEFINED BY A YEAR OF DEMAND, OPPORTUNITY IN MILITARY MEDICINE, AND NATIONAL HEALTH EMERGENCY
A Note from MTEC President Bill Howell

– Membership Growth: MTEC membership continues to climb, we ended 2020 with a record 461 members.
– Research Program Growth: As a result of the pandemic, 2020 was a record year in so many ways, good and bad. We reached a high mark in awards, totaling nearly $180M last year, about $69M of which relate to COVID-19. We have projected $100M in funding for 2021, expecting that we will not see a repeat of COVID funding. It looks like we likely will exceed that projection, as most of that is in the queue already, just one month into the new calendar year. To match our research program growth, we welcome new MTEC team member, Dr. Gage Greening, who will be working closely with our Research Program Director, Dr. Lauren Palestrini. Great to have you on board, Gage!
– Commercialization Program Growth: The MTEC Board charted important growth for our military sponsors and members in the last quarter of 2020, namely, to grow our capacity to support members developing critical medical technologies needed for our military and civilian populations. This includes commercialization support, subject matter expert networks, start-up coaching and networking, and investor and development partner resources, including close collaboration with ARCH Ventures, led by Corey Ritter. An Investment Committee of the Board identified a core set of strategic growth targets to drive impact for our sponsors, members, and military. Our Director of Commercialization, Rick Satcher, has outlined a monthly webinar series that includes training on how to work with the military and will cover special topics like cybersecurity, investment preparation, regulatory compliance, and best practices. You can find more information about the webinar series here. As an intermediary serving our sponsors and members, we need you, members, to build our network with input on who you are and what is important to you. Rick and the team are beginning to create and publish executive summaries starting with our small business members, to help deliver resources that can position you for commercialization success. Thank you for responding promptly to him. You comprise the majority of our non-traditional membership, over 70% by most recent count! These efforts are important for scouting and impact, and they help to define effective med-tech innovation for the country.
– Industry Partners Program: The MTEC Investment Committee also identified an important growth channel through outreach to our large industry partners. We will begin targeted discussions with you this quarter to learn about your priorities, offerings, new markets, and collaboration opportunities, including possible teaming within the MTEC family to improve the likelihood of success in accelerating health innovation for our sponsors. Where 2020 gave us challenges, 2021 brings these new opportunities. We stand ready to make the best of them with and for you.
– Foundation Relations and Fundraising: The MTEC Investment Committee also identified an important growth channel through outreach to our large industry partners. We will begin targeted discussions with you this quarter to learn about your priorities, offerings, new markets, and collaboration opportunities, including possible teaming within the MTEC family to improve the likelihood of success in accelerating health innovation for our sponsors. Where 2020 gave us challenges, 2021 brings these new opportunities. We stand ready to make the best of them with and for you.
ACTIVE SOLICITATIONS
21-05-Cross-cutting – Comprehensive Cross-Cutting Prevention Opportunity to Decrease
Harmful Behaviors and Increase Service Member Readiness and Performance
- This Request for Project Proposals is focused on optimizing health promotion via prevention initiatives for the military that provide education and skills, protective environments, and healthy climates and relationships in efforts to prevent various forms of violent, abusive, or harmful behaviors.
- White Papers are due on February 11, 2021.
Far Forward Burn Treatment (FFBT)
- Development of treatments for severe burn injuries (burn conversion prevention, infection prevention, non-surgical debridement, and temporizing coverings) in a far-forward, austere environment to address the harmful sequelae of the burn injury during prolonged care, which could extend for several weeks post-injury
interoperable Medical Command
and Control System – Joint (iMCCS-J)
- Focused on the development of a prototype that supports the integration of medical data (medical unit locations, unit capacity status, medical evacuation unit capacity status, and patient status) with the Air Force Research Laboratory, Tactical Assault Kit suite of software and hardware in support of iMCCS-J
Military Prototype Advancement Initiative (MPAI)
- Aims to solicit for a broad range of medical prototype technological solutions [medical techniques, knowledge products, and materiel (medical devices, drugs, and biologics)] related to prolonged field care, medical readiness, emerging technologies, and maximizing human potential
interoperable Algorithms
for Care and Treatment (iACT)
- Aims to provide soldiers and military medic personnel with artificial intelligence (AI) and machine-earning based decision support software to enhance their ability to provide short and long-term patient care in all domain operation (ADO) environments
The following topics have not been officially pre-announced but have the potential to become active funding opportunities in 2021 (subject to change):
interoperable Field Hospital (iFH)
- Development of a prototype to create a deployed interoperable field hospital environment that will integrate existing Department of Defense (DoD) tactical networks, systems and medical devices via a Local Area Network (LAN) within deployed Medical Treatment Facilities (MTF)
Enterotoxigenic Escherichia Coli (ETEC) Care
- Medical technology and solutions that support the prevention of ETEC infection
- Solutions that treat and/or cure ETEC
Combat Casualty Wound Care
- Wound care treatments / therapies for combat injuries
- Innovative wound care technologies to treat and prevent biofilm formation
Human Performance
- Body-worn devices that detect changes in physiological conditions associated with the onset of physiological episodes (PE) including a risk alert to the user during tactical performance in time to implement corrective actions
- Enabling capabilities that increase the effectiveness and fieldability of PE detection and alert systems including, but not limited to, physiological risk profiles, specialized device hardware components, improved wearability
- Pre- and post-exposure treatments or biotechnology solutions that enhance / restore operator performance or reduce operator impacts /susceptibility from / to PE conditions, thus maximizing operator resilience / recovery
If you have any questions, please reach out to Dr. Lauren Palestrini, MTEC Director of Research.
Awards
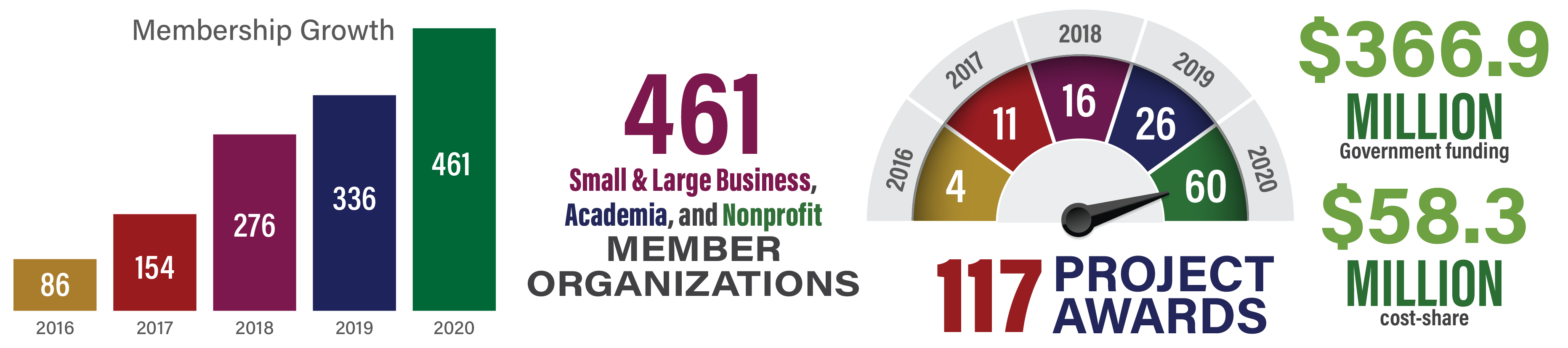
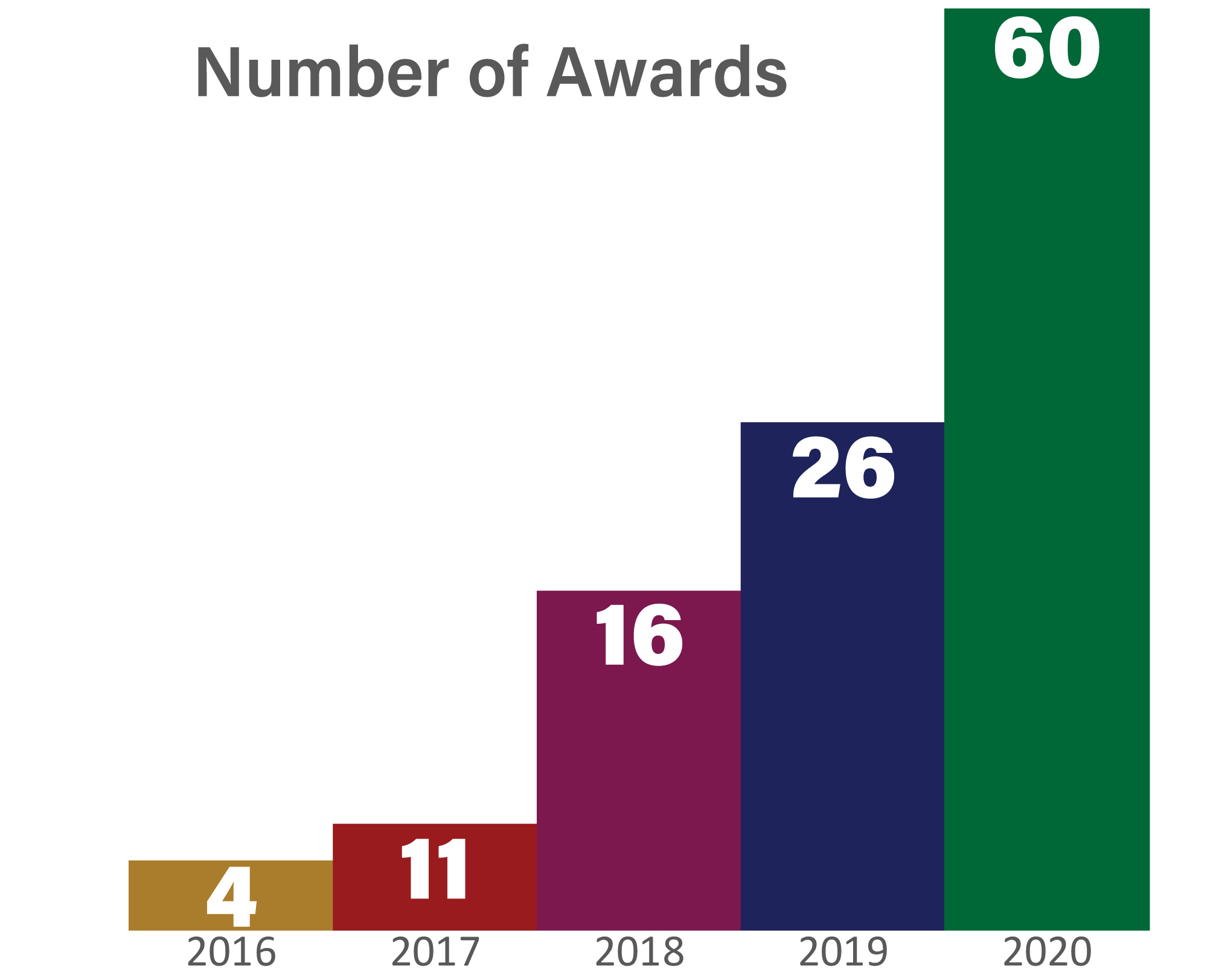
2020 was a year of tremendous growth for MTEC. We now have 117 projects on award. To view the complete list, click here. We are grateful to our government sponsors who continue to look to the MTEC Consortium to find solutions.
Project Highlights
2021 is off to a great start! More and more MTEC projects are beginning to achieve major milestones in their work plans. Here is a sampling of MTEC project highlights from the last quarter of business.
Reduction of HIV Prevalence and Incidence Using TIPs
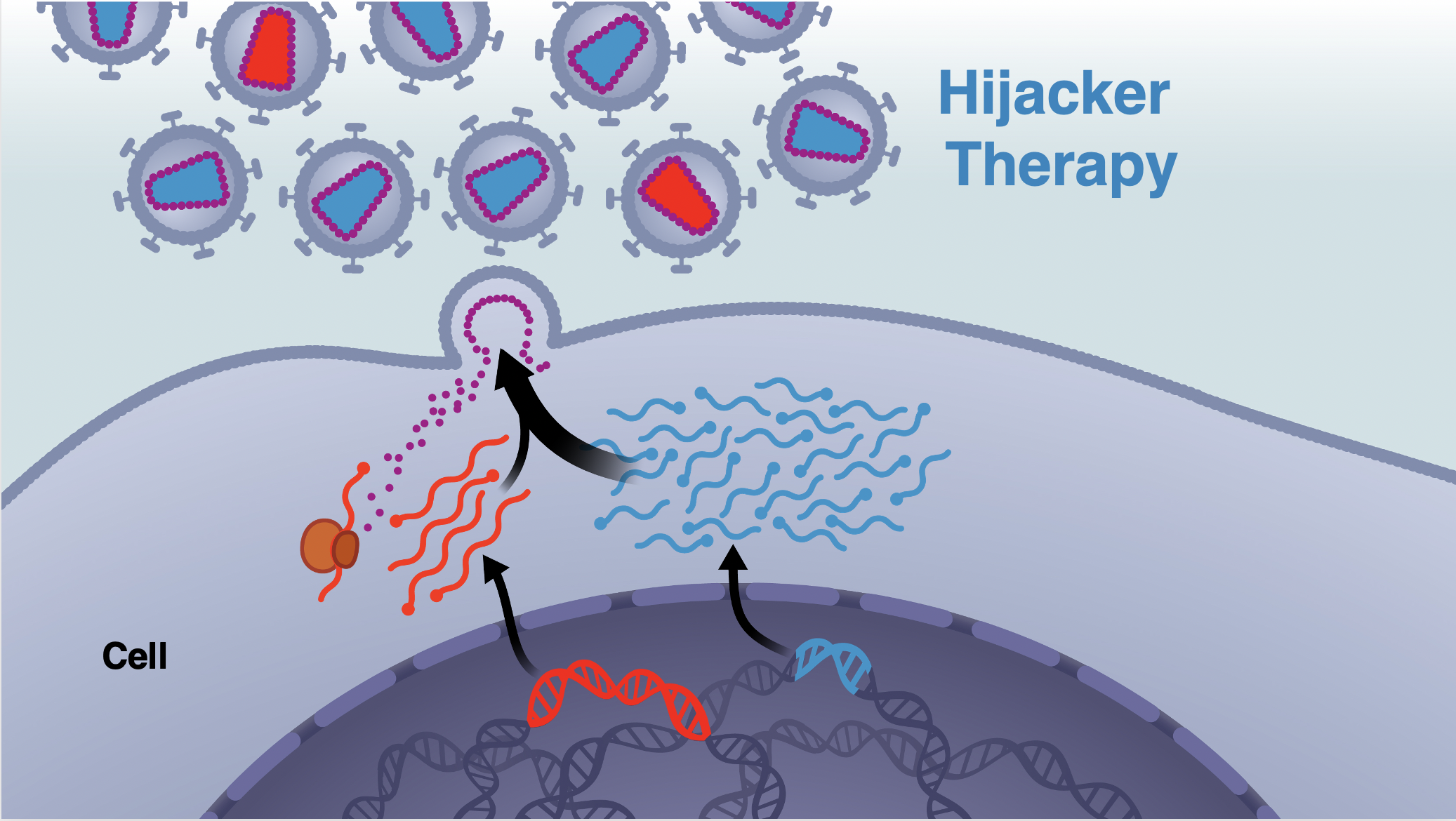
The Weinberger lab at the Gladstone Institutes at the University of California, San Francisco has developed a novel therapeutic paradigm to reduce HIV prevalence and incidence. The approach, named Therapeutic Interfering Particles (TIPs), are engineered, synthetic deletion variants that outcompete HIV for essential proteins within infected cells and substantially reduce HIV burden in vivo to halt viral transmission and disease progression. With MTEC funding, the team recently demonstrated TIP safety and efficacy. In addition, the U.S. FDA provided initial approval for Phase-I clinical trials on these data.
Deployable Bioreactors to Produce Platelet-like Cells
Platelets are the principal blood cell responsible for clot formation and blood vessel repair at sites of active bleeding. Platelet transfusion following severe trauma is associated with improved survival. PlateletBio has been successful in producing PLCs directly from thawed megakaryocytes (bone marrow cells responsible for the production of platelets) in a bioreactor. Additional process improvements have been made, while the design work for the scaled-up bioreactor has been finalized as well as the tech transfer for injection molding. Work has been completed on the software development and the process diagram. These uniform batches of PLCs would last the full 10-day life span in storage as they are freshly made and are manufactured from a sterile process. PLCs could serve as an alternative reliable source for cold-stored, frozen, or lyophilized platelet storage technology that could revolutionize field care.

Wearable Diagnostic for Very Early Symptomatic Detection of COVID-19 Infection

Philips North America, LLC has created a public-private partnership with BioIntelliSense, the University of Wisconsin, eTrueNorth, the U.S. Department of Health and Human Services (HHS), and USAMRDC. This MTEC project led by Philips is funded to “recruit 2,500 volunteers from 22 testing sites located at the University of Wisconsin facilities. Participants in the study will be shipped BioIntelliSense’s BioSticker medical device (with its BioHub wireless gateway) that will be worn for 14 days to continuously capture and monitor the participants’ temperature, respiration rate at rest, heart rate at rest, sleep/rest/active state, body position, and/or coughing frequency. To read the full article, click here. This information will be used to determine the device’s effectiveness for detecting and monitoring COVID-19 infection. The deliverable at the end of the period of performance is to obtain Emergency Use Authorization for the wearable capability and be ready to distribute the device. This clinical trial is now actively recruiting participants.
Engineered Antibody Neutralizes Pandemic Coronaviruses
Centivax, Inc. is developing an optimized antibody therapeutic to treat as well as protect against COVID-19. With support from the U.S. Army Medical Research and Development Command’s (USAMRDC’s) Military Infectious Disease Research Program (MIDRP) and MTEC, Centivax was able to successfully engineer a potent therapeutic antibody in less than 9 weeks, as well as conduct the relevant animal and toxicology studies. Centivax is currently in manufacturing and is preparing to begin clinical trials in 2021.
To enjoy the previous highlights, click here. If you have any questions regarding these research efforts, please reach out to Dr. Lauren Palestrini, MTEC Director of Research.
EDUCATION, MENTORING AND COMMERCIALIZATION
MTEC Webinar Series
The 2021 MTEC Webinar Series will provide monthly events to help our members learn about a broad array of topics to help them develop and commercialize their technology. We will include a 4-part series on how to work with our military, starting with our Jan 26th event, “Understanding the military customer, Part 1 – What does a good proposal look like?” This webinar will provide an overview of technical requirements and present insights into the factors that go into proposal evaluations. Panelists will also describe common technical, budgeting, and contracting challenges they see when reviewing proposals and offer suggestions that may help members craft proposals that address all military needs. Other parts in the series will include insight on how best to manage an award, how to negotiate agreements, and how to get on the procurement list. We will also cover cybersecurity, investment preparation, regulatory and exit strategies.
Here is an overview of our first three webinars:
| Date | Topic | Topic Description |
| Jan 26, 1 pm EST | Understanding the military customer, Part 1 – What does a good proposal look like? | What is the military looking for in proposals and how do you put together a strong one? Key challenges among proposers and key factors for determining success. |
| Feb 23, 1 pm EST | Cybersecurity | Federal Marketplace Update – “CUI, NIST and CMMC” |
| March TBA | Collaboration Opportunities with Military Laboratories –Showcase of several military laboratories with opportunities for collaboration with the MTEC network | Showcase the military sponsors and MTEC mission in light of the OTA – how do we work and how does MTEC benefit members? |
MTEC Mentoring and Commercialization Services
As part of our Member Connect initiative, we are looking into the option for an online collaboration tool where we can create discussion channels dedicated to upcoming solicitations, services, and other topic areas of interest. The goal is to enable member-to-member discussions, and interactions with MTEC staff, that can help team for proposals, find assistance for research and development activities, and learn more about other members. We are excited to see how this new communication tool can help add value to your membership.
As we move into 2021, MTEC is expanding commercialization services for its members. We have several key initiatives that we think will help advance your technology to market. We are planning to offer support that will help you find additional funding, further develop your team, and find technical and business expertise needed to advance your technology. We’ve started partnering with funders such as ARCH Ventures and other enablers to assist you. The network will continue to grow.
To start this process, we are working with our small business awardees to complete an executive profile. The profile provides an overview of your technology, applicable patents, your MTEC project, and other key discriminators. Our partnership with Life Science Intelligence (LSI) gives us the ability to submit profiles of our member companies, with your permission, of course, for review by potential investors. The LSI database is a proprietary, vetted compilation of hundreds of medical technology investors. These profiles will also be provided to our military sponsors and other sources of non-dilutive capital with your permission. We plan to build them into our Member Connect website for other members to see who you are. This way we are giving you maximum exposure for your technology as a free MTEC Member Benefit.
– Rick Satcher, MTEC Director of Commercialization
OUTREACH
MTEC Foundation Relations Program
The MTEC Board also helped to define a renewed focus on nonprofit and foundation relations. We appreciate that military funding is finite, and that most of our life science product development requires additional funding. Foundations can play a critical role here, in complement to our core mission.
According to data released by Giving USA, philanthropic giving by foundations represented 17% of overall giving in the United States in 2019, totaling approximately $75.69 billion. Foundations represent an important network of knowledge building and potential funding, both for MTEC awards and MTEC members. In 2021, MTEC seeks to engage medical research foundations in ways that can ultimately further mutual research objectives. There are different forms these partnerships can take.
One partnership avenue is the exchange of information on research award opportunities, such that MTEC members are knowledgeable about relevant foundation RFPs and aligned foundation grantees/researchers are aware of upcoming MTEC RFPs. This can help to ensure strong solicitation responses across the broad networks that each entity maintains.
Those foundations that become MTEC members will have access to the many benefits of MTEC membership, including networking, dialogue with the military R & D community, knowledge of upcoming military research priorities, commercialization support services, and educational services that can benefit their grantees.
And, finally, there is opportunity for the foundation to co-fund medical research with MTEC’s military sponsors, leveraging foundation dollars with public dollars for greater impact.
We look forward to keeping you appraised of our foundation relations throughout the year. For additional information, please contact Kate Golden at [email protected].
Events
Upcoming Events
MTEC Webinar Series: Cybersecurity
February 23, 2021 1pm EST
- Federal Marketplace Update – “CUI, NIST and CMMC”
- More information and registration coming soon!
- MTEC is hosting a virtual conference focused on accelerating modernization to enable multi-domain operations.
- To view the tentative agenda, click here.
- Registration will open in February— Mark your calendar now to be part of this exciting event and connect with potential partners and funders!
Recent Event Highlights
Redefining Early Stage Investments (RESI) Conference
November 17-19, 2020
- MTEC was excited to participate in the 1:1 Partnering Sessions.
- RESI is committed to facilitating conversations that spark early-stage deals in across drugs, devices, diagnostics, and digital health that save lives and change the world through healthcare innovation. To advance the most successful deals, all major players, including both buyers and sellers, are saved a seat at the table. In the past 7 years, over 300 companies have raised $400+ MM through 30 RESI Conferences. RESI attracts incubators, tech transfer offices, universities, hospitals, research labs and their constituents getting on the radar of investors and channel partners. Biotech, medtech, diagnostics, and digital health companies source global investors and strategic partners, create relationships, and potentially, secure funding from Seed to Series B.
Webinar Series Part 1: How to Write a Good Proposal
January 26, 2021 1pm EST
- Understanding and responding to the technical requirements for a Request for Project Proposal (RPP) from the Army is critical to receiving an award. This webinar will provide an overview of technical requirements and present insights into the factors that go into proposal evaluations. Panelists will also describe common technical, budgeting, and contracting challenges they see when reviewing proposals and offer suggestions that may help members craft proposals that address all military needs.
Moderator:
Lauren Palestrini, MTEC Director of ResearchPanelists:
Kimberly Pope, CDMRP
Jeff Bartlett, USAMRAA
Thomas Dunn, Naval Advanced Medical DevelopmentMTEC Members can find slides and speaker notes from the webinar on the Members Only site.










