This week, the MTEC was at the Military Health Systems Research Symposium (MHSRS) hosted at the Gaylord Palms Resort in Kissimmee, Fl. We were fortunate to have a booth large enough to host a small poster session as an extension of the larger MHSRS poster session. Over the course of three days, we rotated through 42 different MTEC members presenting the latest data and advancements for their products. We appreciate all of the presenters taking the time to walk us through their exciting product developments. Several of these presentations can be found below, divided into the following sections:
Section 3: Human Performance and Regenerative Medicines
Section 4: Infectious Diseases
Section 5: Simulation and Training
We are grateful for all of the MTEC members that participated at MHSRS. We look forward to seeing you all again next year!
Section 1: Brain Health
Section 1: Brain Health
“Handheld Brain Diagnostics”
Infrascanner is the only handheld device in service that can detect brain hemorrhage at near 95% sensitivity and specificity levels. Partnering with USAMMDA and MTEC, Infrascan is developing the next generation of this technology to be available for use by the Marine Corps and all services, as well as funding research toward potential detection of brain hemorrhage expansion.
NoMo Diagnostics and Rhythmlink International
“Wearable brainwave sensor system for real-time injury detection”
NoMo Diagnostics has developed a miniaturized brainwave monitoring system (qEEG) that allows for real-time detection of potential mTBIs. Through MTEC and M-Corps collaborations NoMo Diagnostics and Rhythmlink International, LLC have entered into a manufacturing and product development agreement to ultimately protect our most valuable asset, the warfighter.
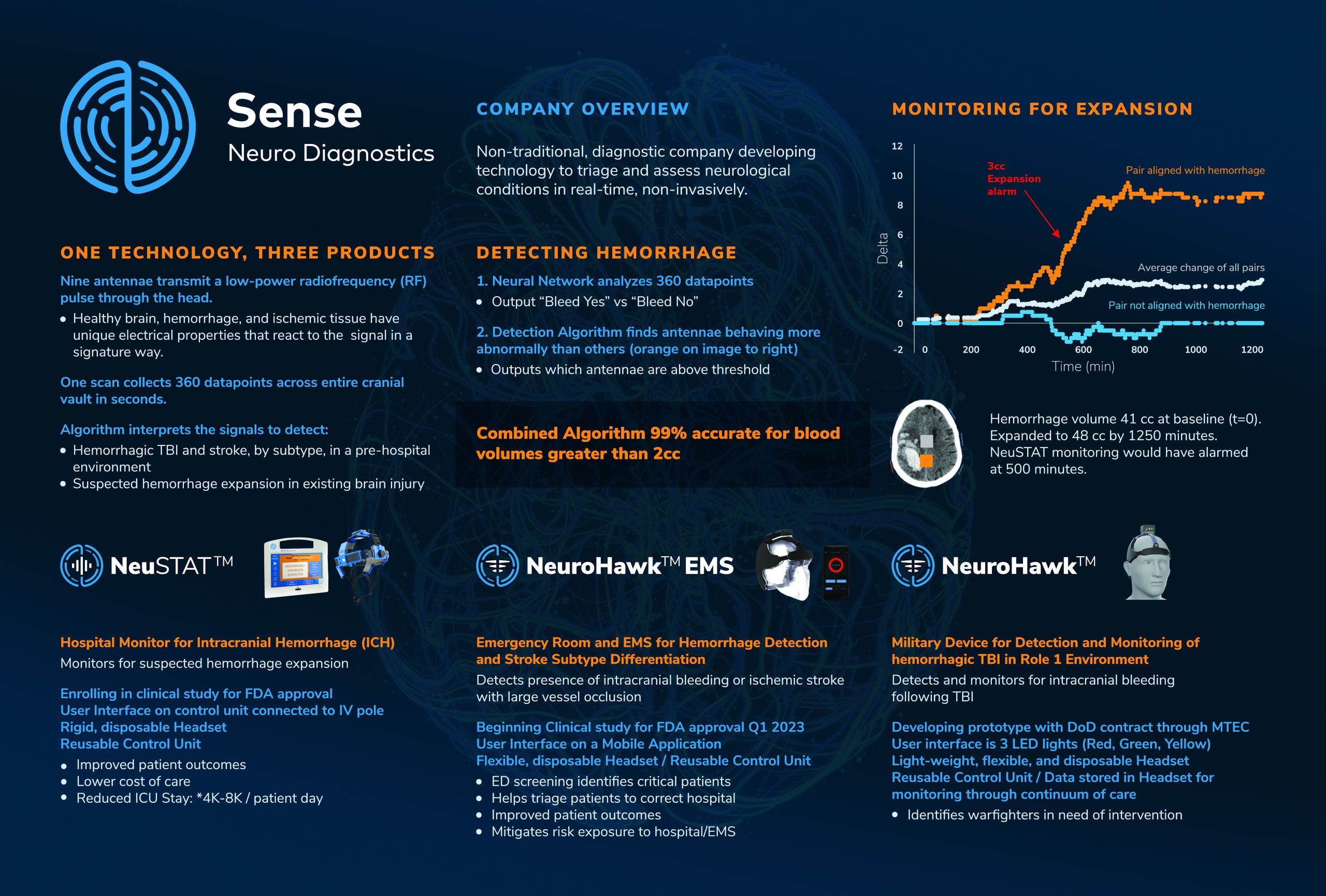
“Sense Neuro Diagnostics”
Sense Neuro Diagnostics is developing a non-invasive brain scanner that can assess and monitor the neurological status of a patient in real time. Our current focus is on detecting and monitoring TBI and stroke.
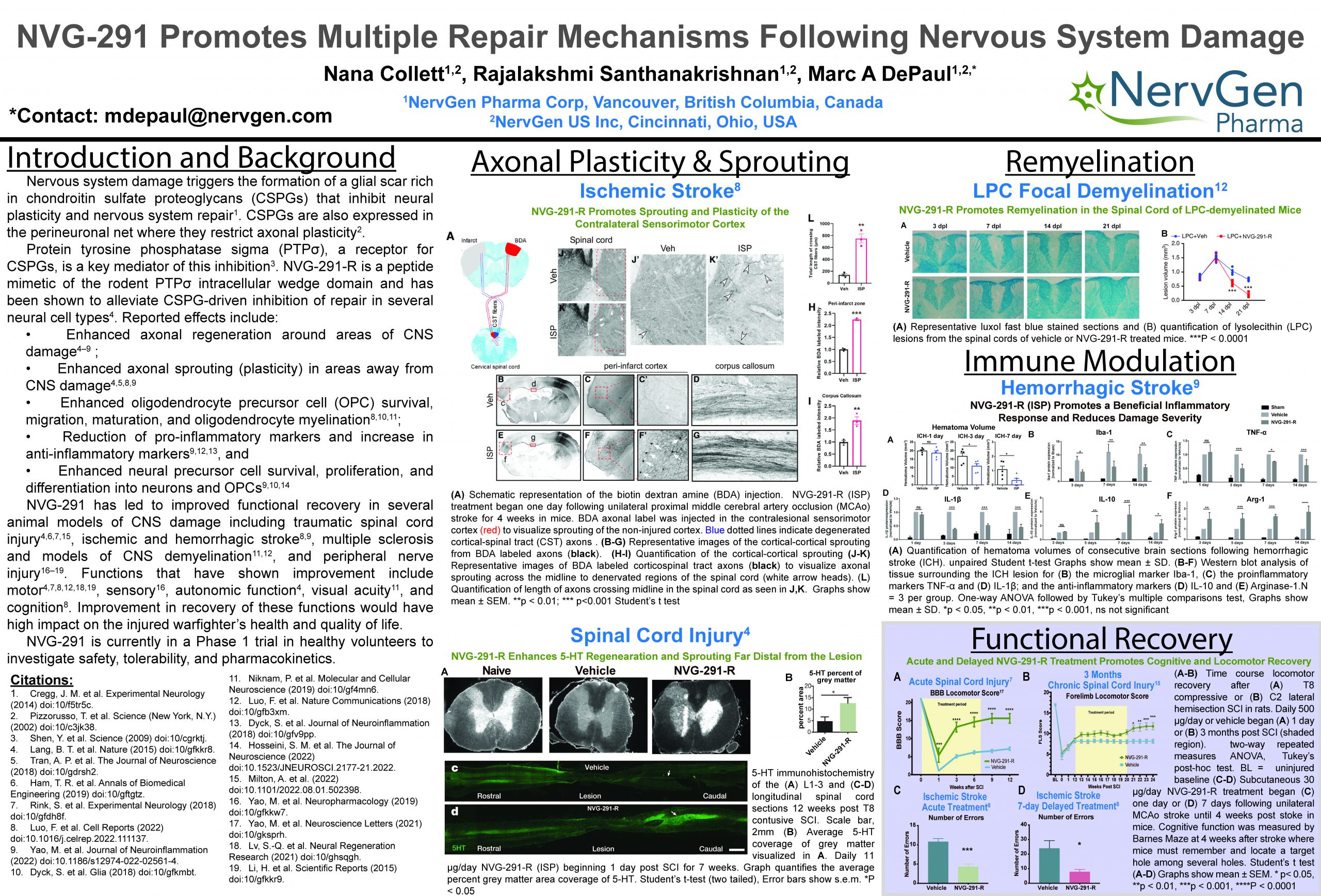
“NVG-291 Promotes Multiple Repair Mechanisms Following Nervous System Damage”
NervGen’s lead drug candidate, NVG-291, enables nervous system repair by relieving the inhibitory effects of chondroitin sulfate proteoglycans (CSPGs) which are upregulated following nervous system damage. NVG-291 promotes functional recovery by enhancing axonal regeneration, plasticity, and myelination, and modulating the immune response to dampen secondary injury.
Section 2: Hemorrhage, Blood
Section 2: Hemorrhage, Blood
“Extending the Reach of the Armed Services Blood Program”
For Traumatic hemorrhage, time to transfusion is critical for reducing mortality. An ideal blood logistics system would deliver blood to theater quickly and move forward with the front line.
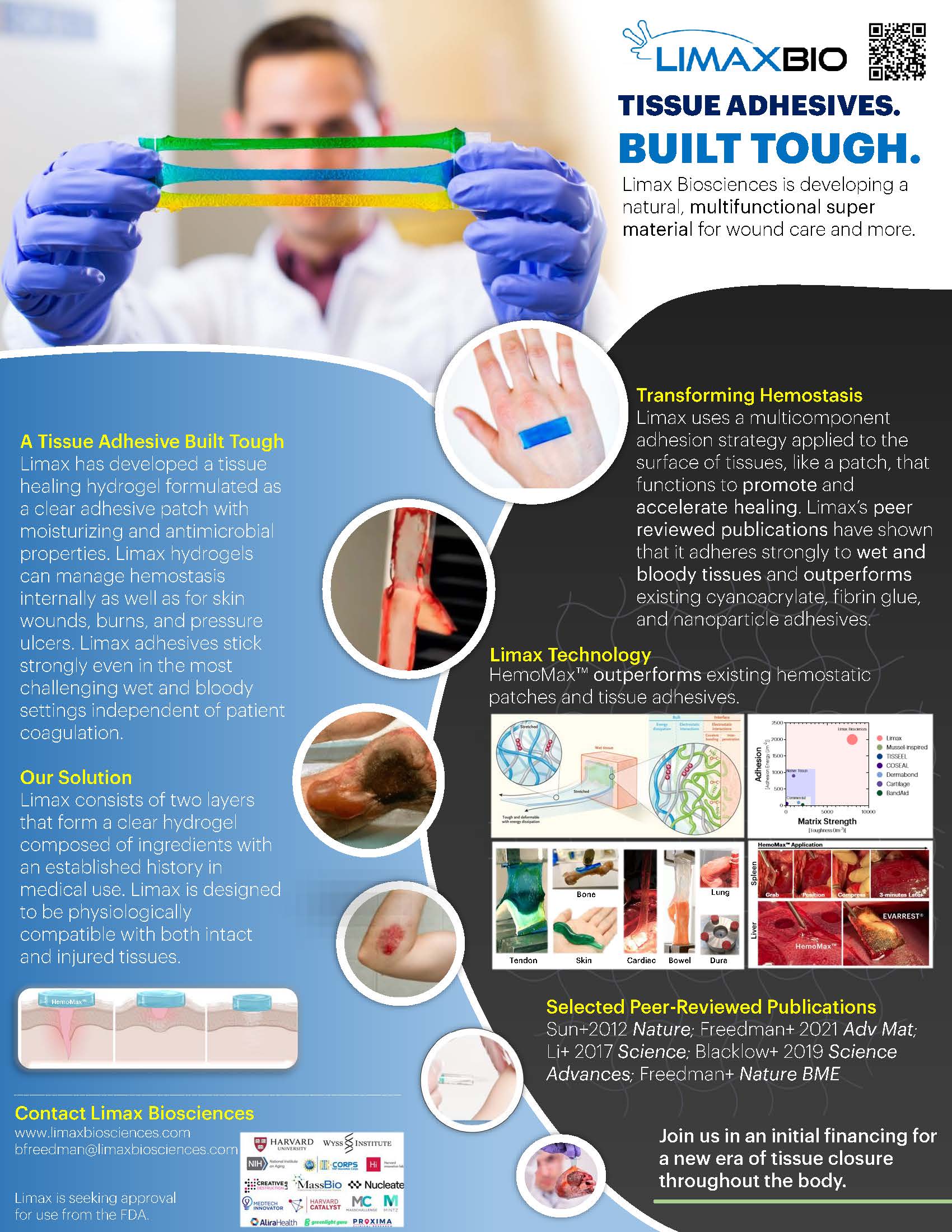
“Tissue Adhesives. Built Tough.”
Limax Biosciences has developed a next generation, stretchable hydrogel-based tissue adhesive to revolutionize treatments for injuries inside and outside the body. Existing hemostatic dressings and sealants have weak tissue adhesion that result in leaks, tissue rigidity, and cytotoxicity. Limax’s degradable, hydrogel-based material addresses these limitations through strong and rapid adhesion to wet tissue surfaces at 10-100x higher the strength of competitors. This patent-protected platform technology can be used in several indications throughout the body including soft tissue reinforcement and local drug delivery.
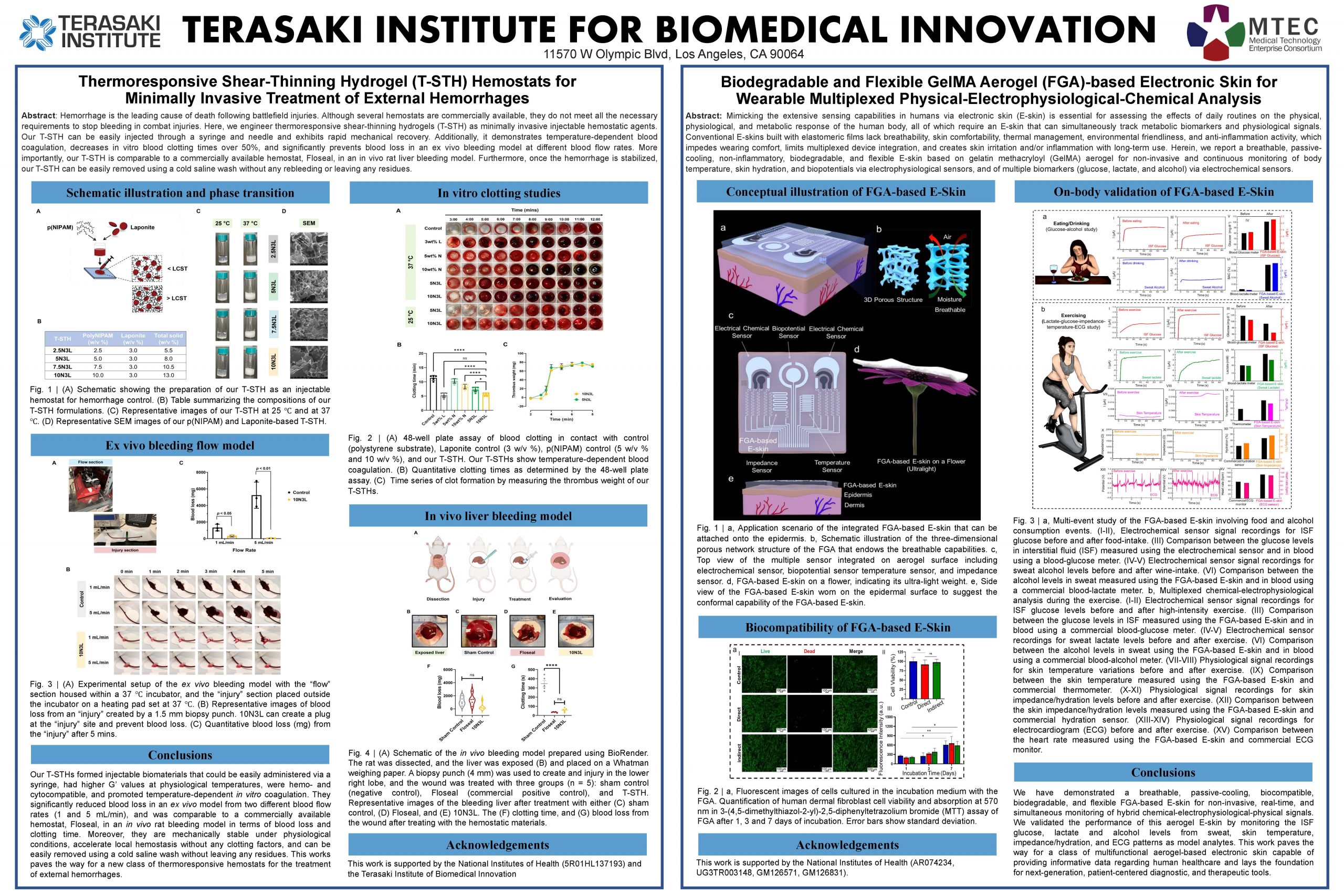
Terasaki Institute for Biomedical Innovation
“Thermoresponsive Shear-Thinning Hydrogel (T-STH) Hemostats for Minimally Invasive Treatment of External Hemorrhages”
Our thermoresponsive shear-thinning (T-STH) hydrogels are engineered for use as as a first-aid in the treatment of external hemorrhages from battlefield and traumatic injuries, and can prevent bleeding without the use of expensive clotting factors. Once the patient has been safely transported to a medical treatment facility, being thermoresponsive, the T-STH ca be easily washed away with cold saline without rebleeding or leaving any residues.
“Biodegradable and Flexible GelMA Aerogel (FGA)-based Electronic Skin for Wearable Multiplexed Physical Electrophysiological-Chemical Analysis”
Our flexible GelMA aerogel (FGA)-based electronic skin is a breathable, non-inflammatory, biodegradable, and wearable electronic skin developed to continuously and simultaneously monitor metabolic biomarkers and physiological signals from the human body.
Section 3: Human Performance and Regenerative Medicines
Section 3: Human Performance and Regenerative Medicines
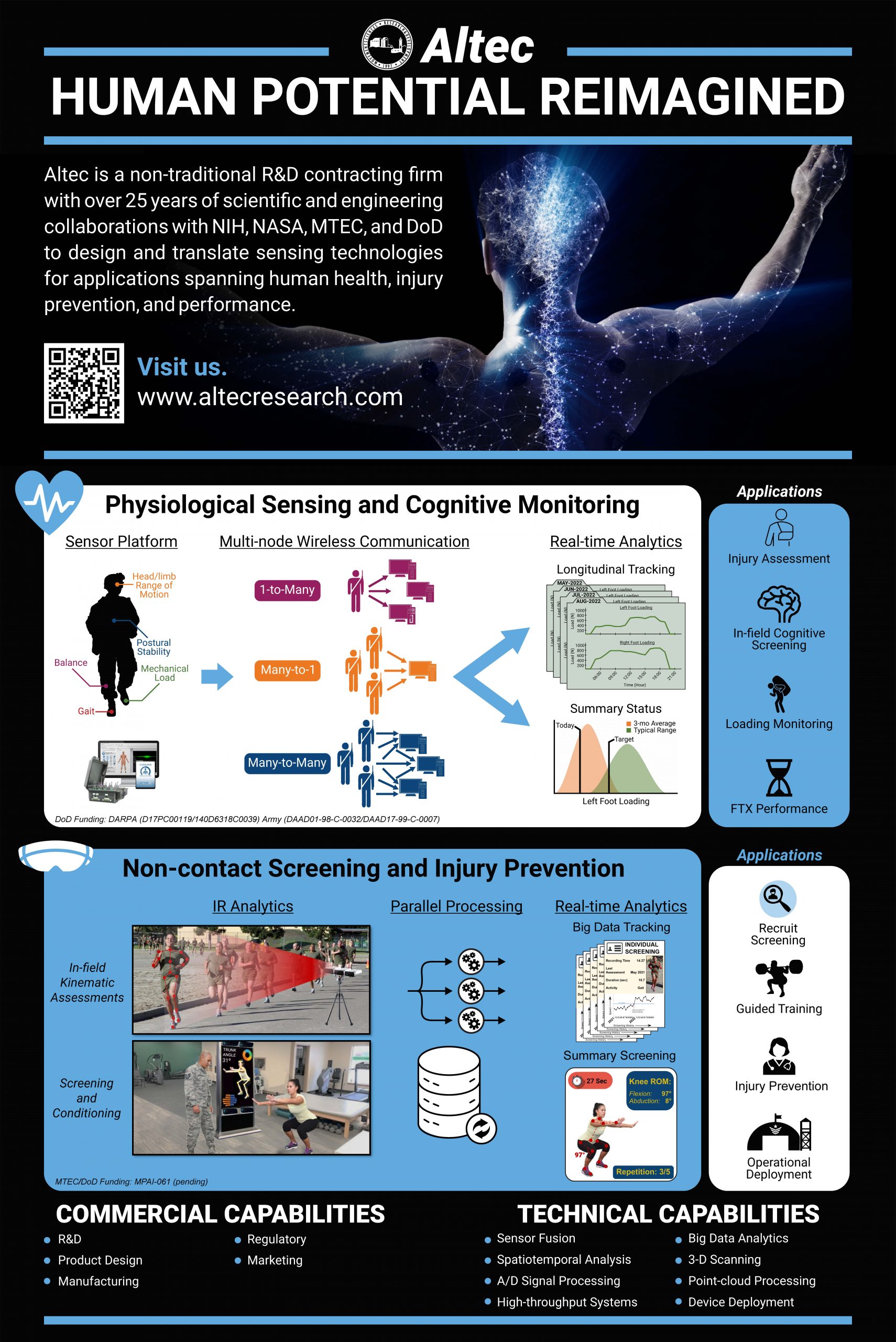
“Human Potential Reimagined”
At Altec we collaborate with a team of scientists, engineers, and practitioners to solve some of the world’s most challenging problems related health and human performance. Our solutions span applications from physiological sensing and cognitive monitoring to non-contact screening and injury prevention to meet the needs of military, medical, and research customers across the country and globe.
RTI International – Durham, NC
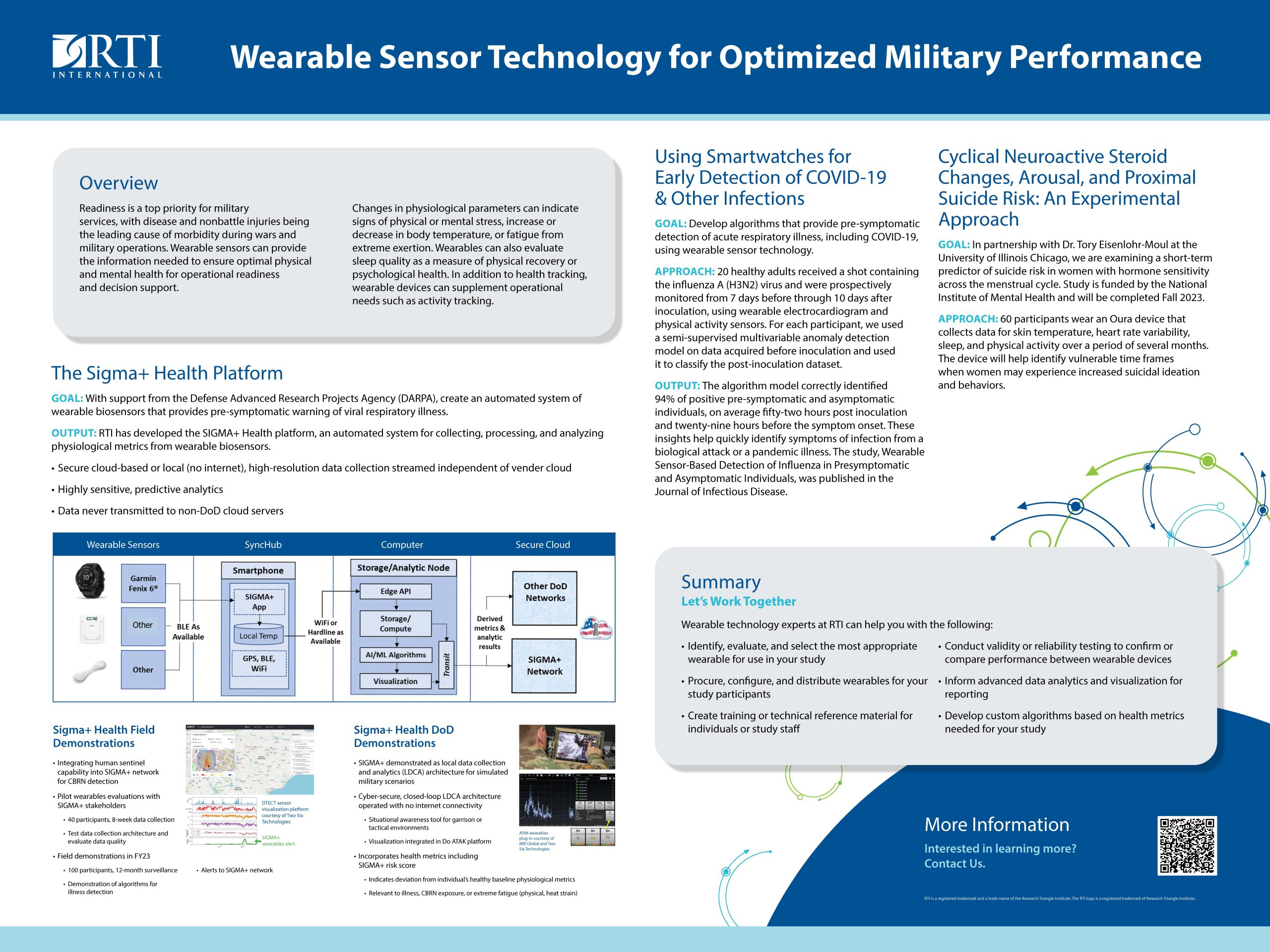
“Wearable Sensor Technology for Optimized Military Performance”
RTI International, with funding from DARPA as part of the Sigma+ Health Platform, developed an algorithm that provides pre-symptomatic detection of acute respiratory illnesses, including COVID-19, using wearable sensor technology. In addition, RTI, in partnership with the U of Illinois, Chicago, is examining short-term predictors of suicide risk in women using wearables.
The Informatics Applications Group, Inc.
“WP2: Wearable Performance Platform”
The Warrior Performance Platform (WP2) enables data management and content delivery to support Human Performance objectives in a scalable and intuitive interface. Current DoD projects include adaptive reconditioning supporting the Army Recovery Care Program as well as dietary and physical activity behavior change for participants in the Navy Delayed Entry Program.
UTL, Inc. DBA Deep End Fitness
“Optimizing Flow + Human Performance with Deep End Fitness (DEF)”
Deep End Fitness is a combat veteran/ service-disabled owned business with a mission to optimize warfighter performance, health, and readiness through aquatic-based training in conjunction with the F.R.E.E. mindset operating system. This approach, developed by former Marine Raiders, enhances mindfulness, develops resiliency, and equips warfighters with proven stress management coping mechanisms to elevate performance under pressure.
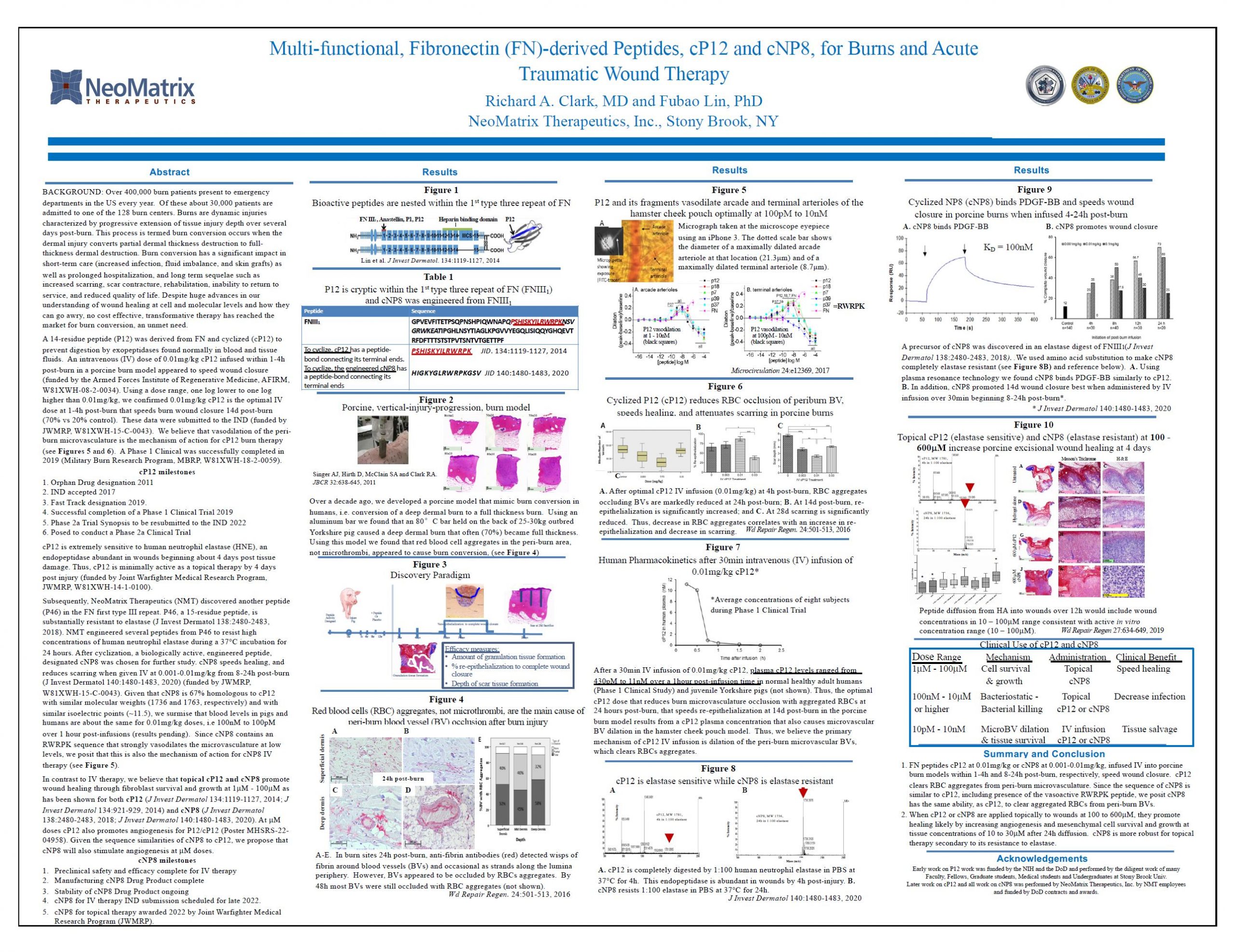
“Multi-functional, Fibronecting (FN)-derived Peptides, cP12 and cNP8, for Burns and Acute Traumatic Wound Therapy”
We are a clinical-stage peptide discovery and development company focused on treatments for unmet medical needs of severe burns and other types of serious tissue injury. We are developing a novel class of fibronectin-derived “epiviosamine” peptides with tunable mechanisms including microvascular dilation, angiogenesis, cell survival and growth, as well as anti-inflammatory and antimicrobial activity.
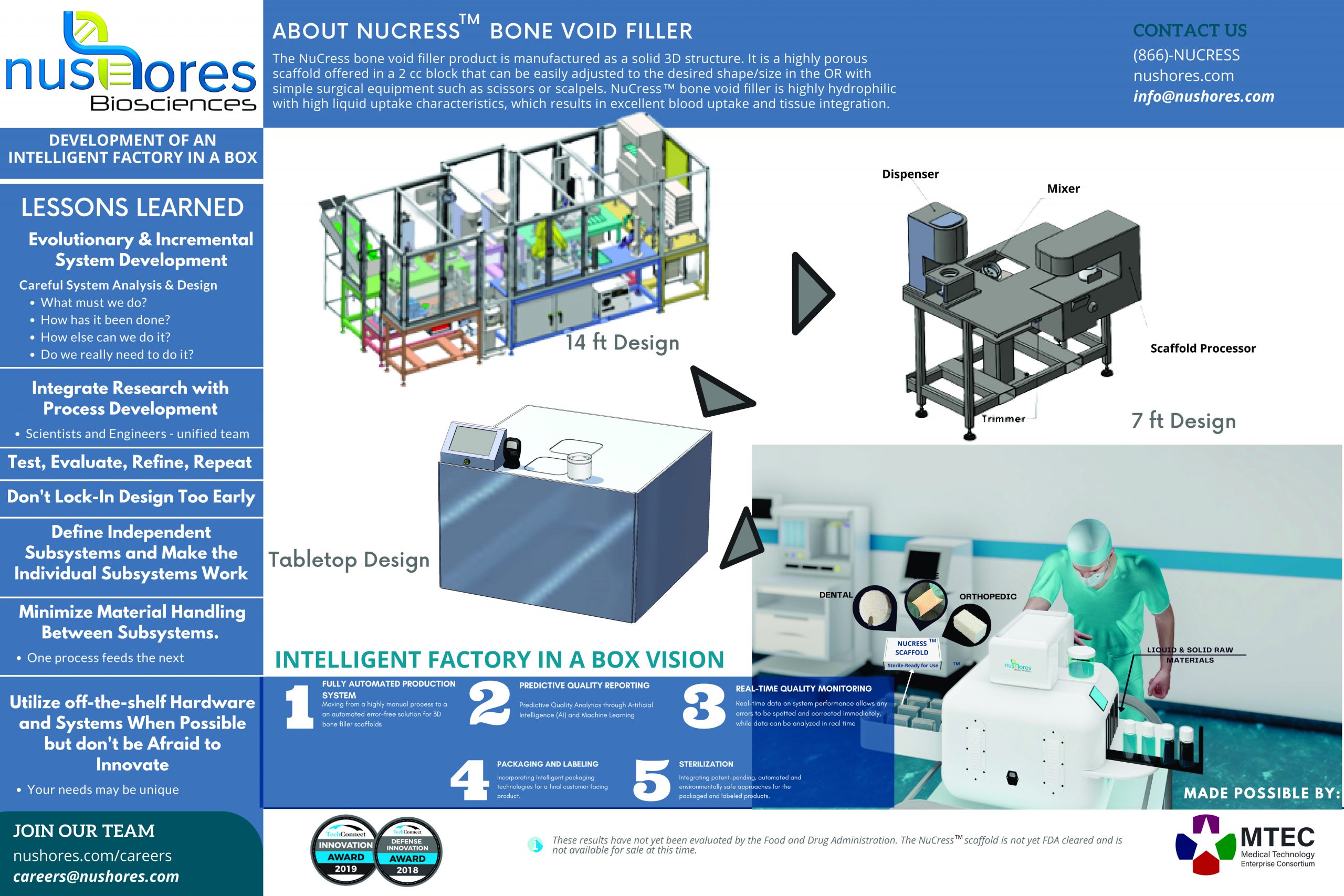
“Development of an Intelligent Factory in a Box: NUCRESS Bone Void Filler”
Our Intelligent Factory in a Box for bone scaffold manufacturing will allow us to transition from a highly manual, highly variable process to a fully automated production system. We envision this system to have minimum operator inputs: providing an error-free solution with predictive quality reporting, real time quality monitoring, and resulting in a ready to use final product that is packaged, labeled, and sterile.
Section 4: Infectious Diseases
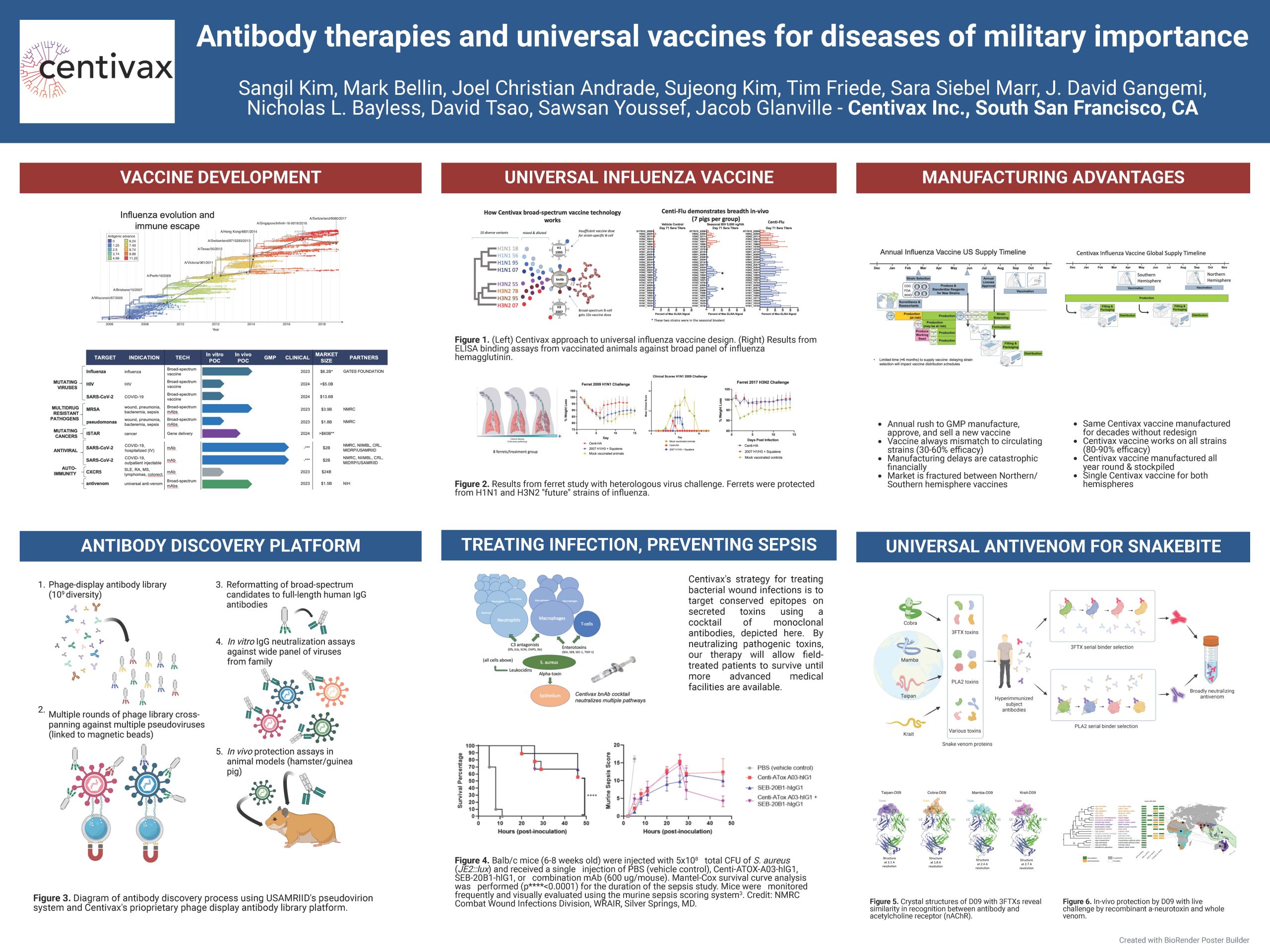
“Antibody therapies and universal vaccines for diseases of military importance”
Centivax engineers thermostabilized broad-spectrum therapies for use in austere environments. Centivax’s computational-guided bioengineering platform enables antibodies to tolerate lyophilization and high concentration formulation, so that these medicines can be deployed as “EpiPen-like” autoinjectors without requiring cold storage or intravenous (IV) delivery. Applications include anti-infectives for wound infection, broad-spectrum antivirals, and broad-spectrum (universal) antivenom. Centivax also develops broad-spectrum vaccines to elicit broadly neutralizing antibodies in vivo against rapidly mutating pathogens.
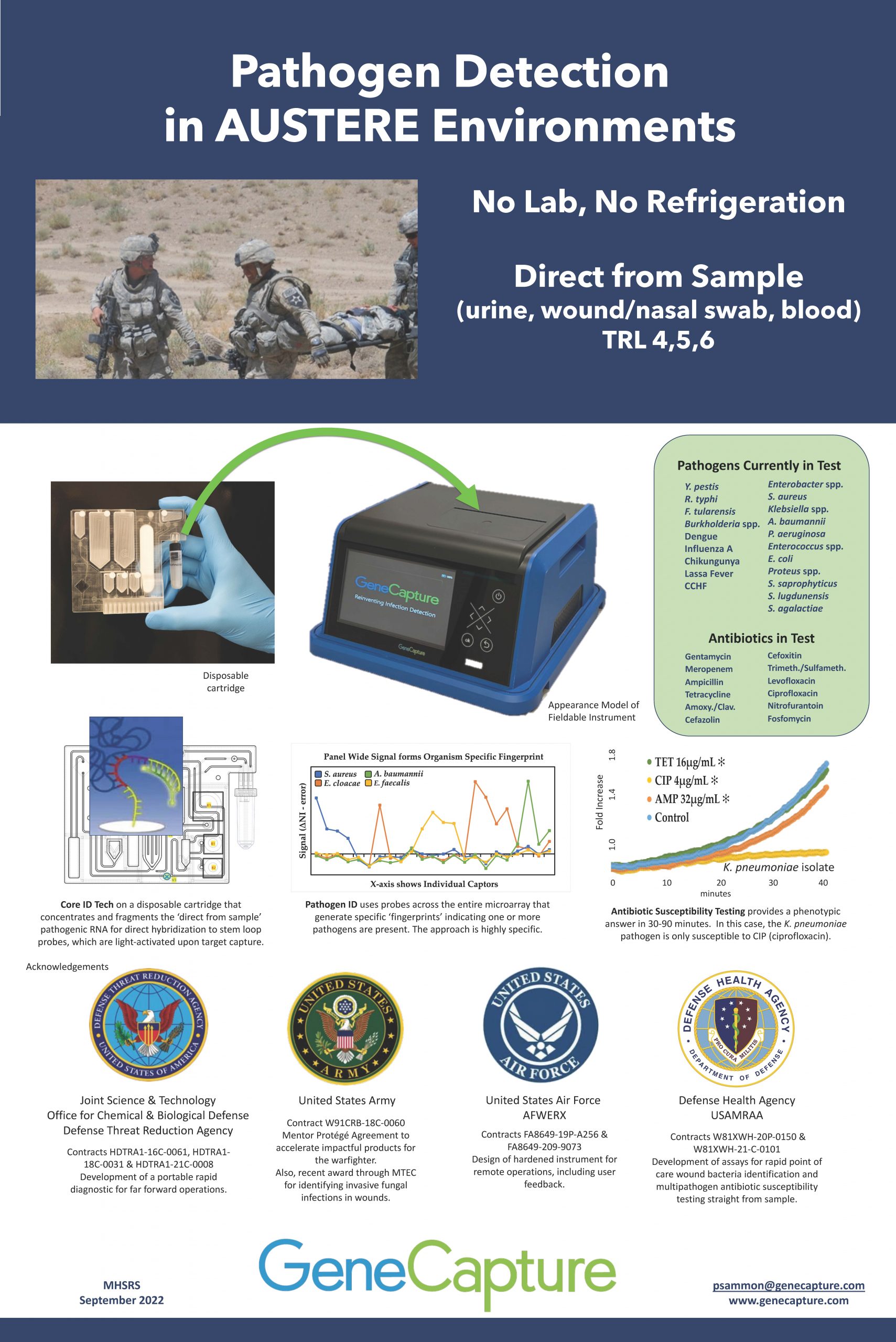
“Pathogen Detection in Austere Environments”
GeneCapture is developing a portable rapid diagnostic for multi-pathogen detection and antibiotic susceptibility for far forward applications. The technology does not require a lab or refrigeration and is designed for ease of use.
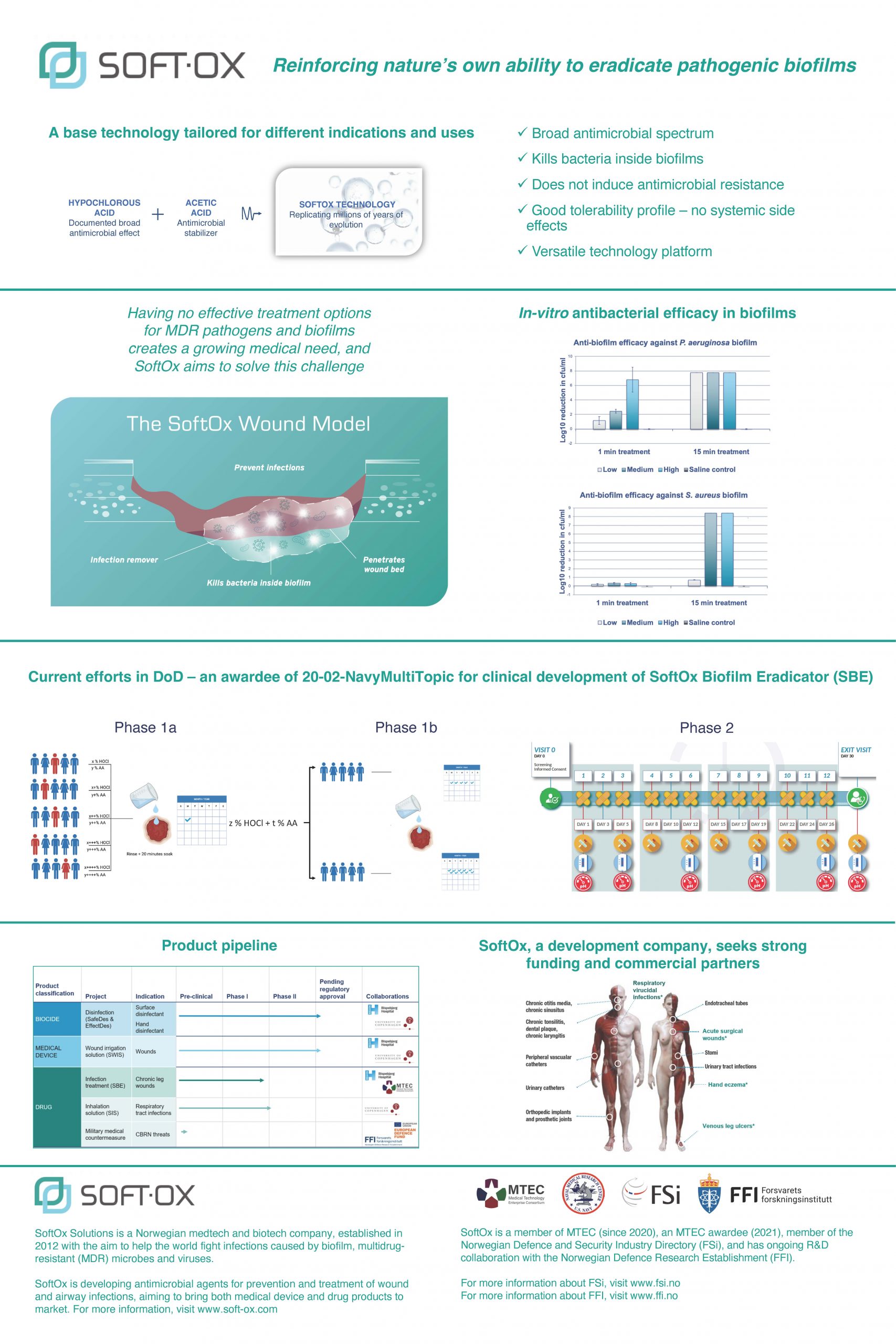
“Reinforcing nature’s own ability to eradicate pathogenic biofilms”
Having no effective treatment options for multi-drug resistant (MDR) pathogens and biofilms creates a growing medical need. SoftOx Solutions aims to solve this challenge by developing topical antimicrobial agents for prevention and treatment of wound and airway infections and bringing these novel products to market.
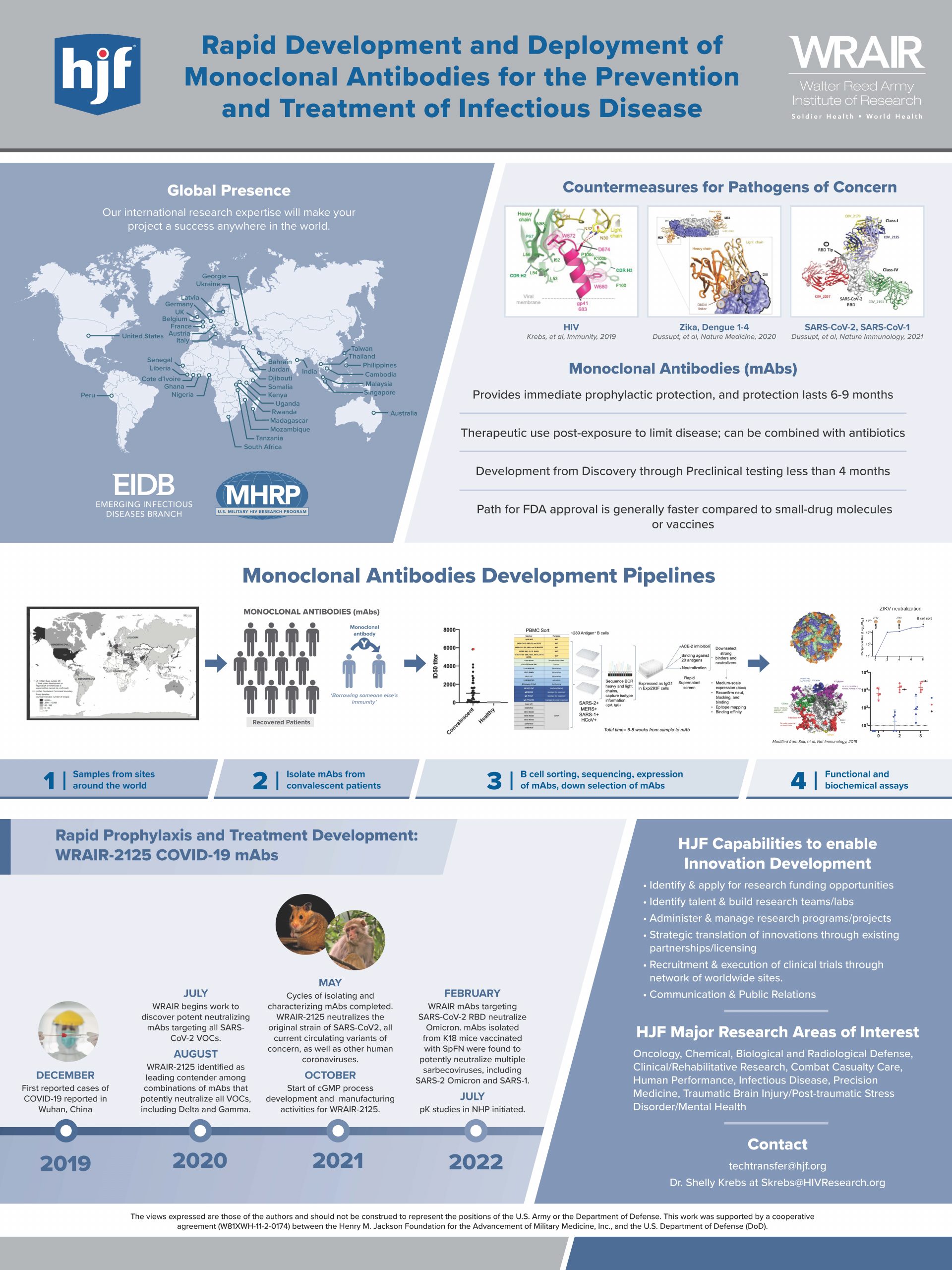
Henry M. Jackson Foundation for the Advancement of Military Medicine
“Rapid Development and Deployment of Monoclonal Antibodies for the Prevention and Treatment of Infectious Disease”
Dr. Shelly Krebs and collaborators have created a novel platform that enables for the rapid development of monoclonal antibodies (mAbs) for the prevention and treatment of infectious diseases. Development of the mAbs from discovery through preclinical testing takes less than four months and utilizes HJF’s extensive worldwide clinical trial network. The mAbs provide immediate prophylactic protection and therapeutic use post-exposure to limit disease.
Indiana Center for Regenerative Medicine and Engineering
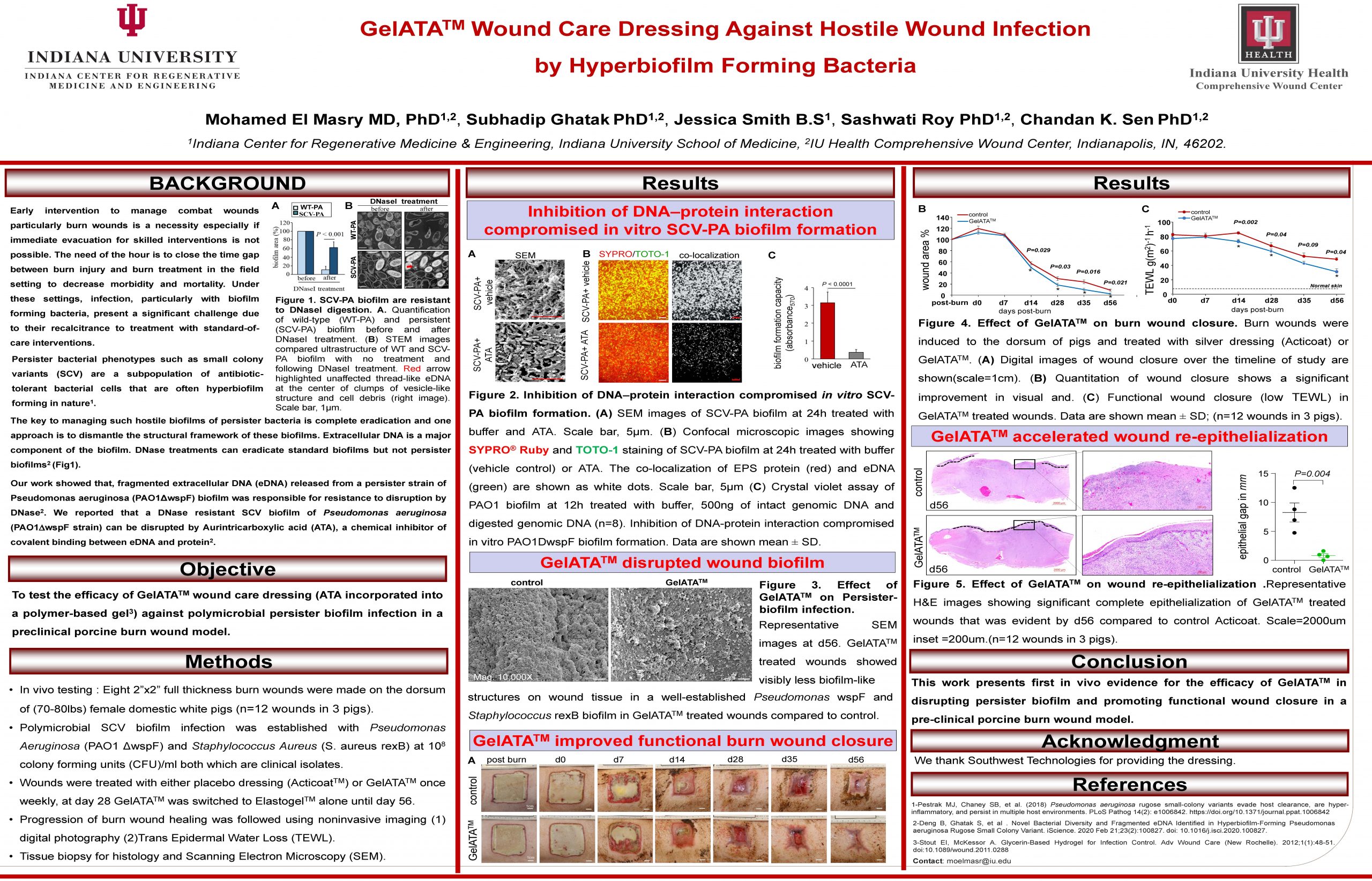
“GelATATM Wound Care Dressing Against Hostile Wound Infection by Hyperbiofilm Forming Bacteria”
Multidrug resistant persister bacterial biofilm infections are difficult to treat. GelATATM is a novel wound care dressing that disrupts polymicrobial persister biofilm and promotes functional wound closure in vivo.
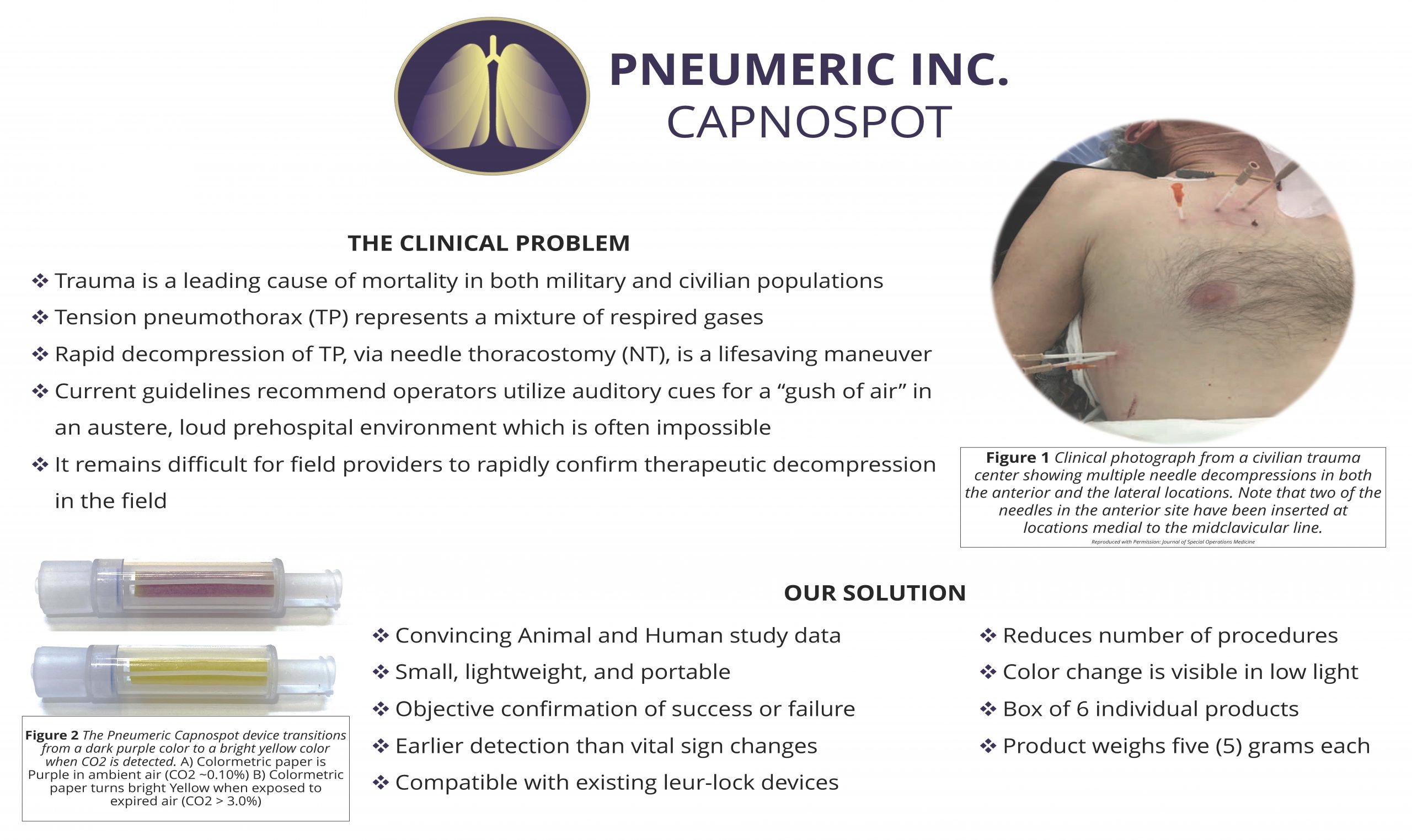
“CAPNOSPOT”
At Pneumeric™, we are on a mission to elevate the level of emergency care with a revolutionary breakthrough in tension pneumothorax care that is poised to transform healthcare for the better. With the groundbreaking Capnospot™ device, emergency providers can now see their results of needle decompression visually – eliminating guesswork, improving patient outcomes, and instantly reducing the number of preventable deaths in the field.
Section 5: Simulation and Training
“AUGMED® eXtended Reality Anytime, Anywhere TCCC Trainer”
AUGMED® is an eXtended Reality (XR) blended training solution for Tactical Combat Casualty Care (TCCC) that combines augmented, virtual, and mixed realities into a next dimension training paradigm. Warfighters can use AUGMED® to train and reinforce TCCC fundamental skills and decision making under realistically stressful conditions by placing the trainee into a virtual, contextualized environment while performing hands-on medical skills application.
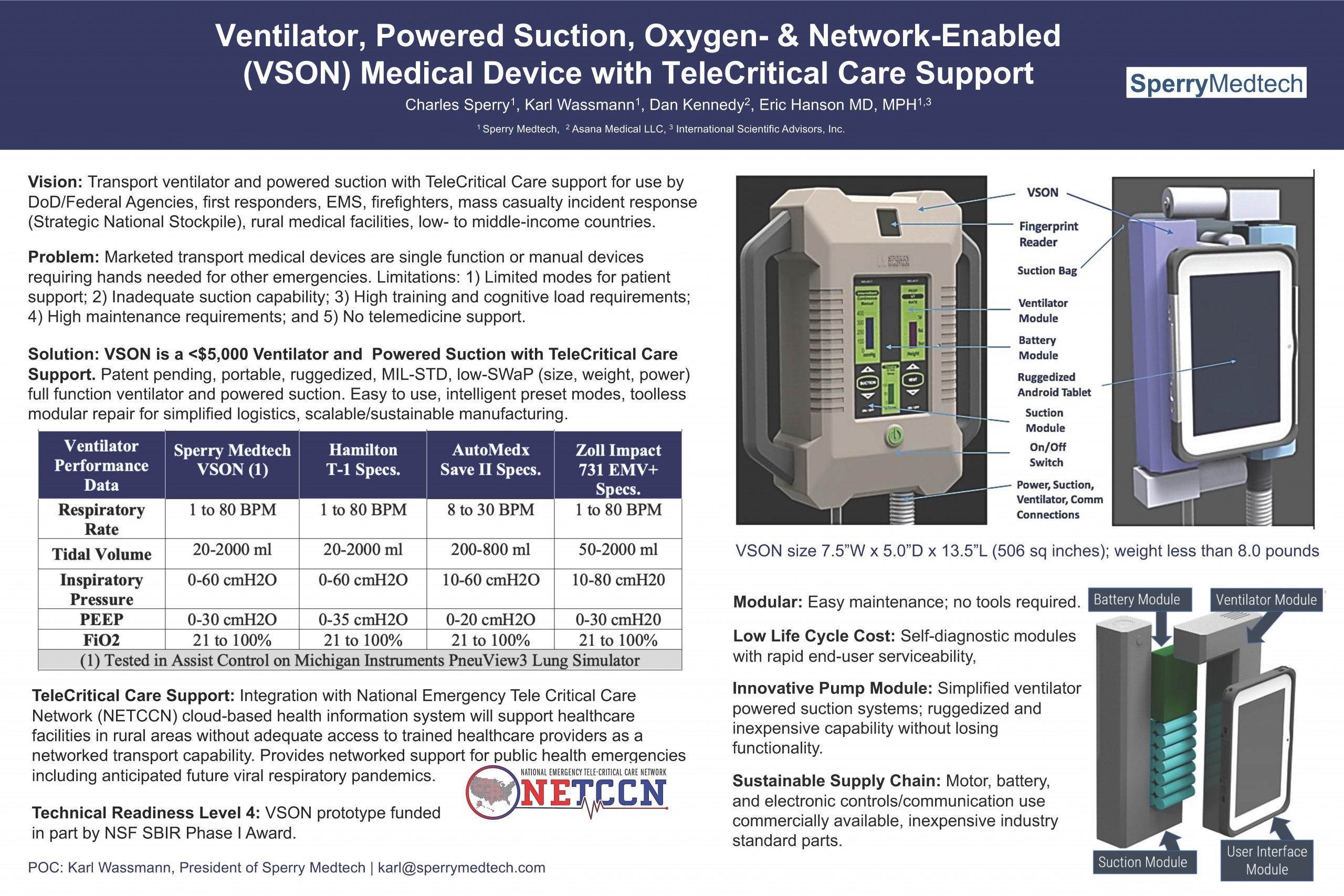
Sperry Medtec
“Ventilator, Powered Suction, Oxygen- & Network Enabled (VSON) Medical Device with TeleCritical Care Support”
Sperry Medtech introduces the Ventilator, powered Suction, Oxygen- & Network-Enabled (VSON) medical device with telecritical care support to be integrated with National Emergency Tele Critical Care Network (NETCCN) cloud-based health information system. VSON is a modular, portable, ruggedized, low cost (<$5,000) transport medical device for support of airway management at point of injury by DoD/Federal Agency personnel, first responders (EMS, firefighters), mass casualty incident response, and low- to middle income country healthcare providers.
“Synthetic Training Environment for Multimodal Medical Training (STEM3T)”
With support from the Medical Simulation & Information Sciences Research Program (MSIS) at the U.S. Army Medical Research & Development Command (USAMRDC), Vcom3D is developing scenario generation tools, assessment instruments, and learning system interfaces that enable the development of multi-modal (live, virtual, manikin) medical training scenarios using the Modular Healthcare Simulation and Education System (MoHSES) standards. These have the potential to lower cost and increase interoperability of medical simulation systems for multi-modal training that can be used by all services at multiple echelons of care.
“Burn Patient Transfer System”
The goal of this program is to research, develop, and test a web based, and mobile app-accessible, open architecture cloud-based system to track capacity and improve the logistics of burn patient/trauma patient triage and transfer in and between military and civilian treatment facilities in the event of a war, disaster, nuclear or other mass casualty with large numbers of burn patients. Following a military or civilian mass casualty or disaster event, military treatment facilities would experience a significant increase in burn patient volume. Burn injuries are rarely isolated events; typically, there is some type of associated trauma along with a burn injury, thus inclusion of trauma bed resources is desirable. There is only one military Burn Unit, the US Army Institute of Surgical Research in San Antonio, TX. While able to expand capacity in the event of a mass casualty event and depending on the geographic area(s) involved in an event, bed capacity could easily be overwhelmed. The ability to maximize efficiency and effectiveness of triage and subsequent care would be critical to the management of an overwhelming surge in burn patient volume and intensity. This effort aims to conduct research and development of a burn/trauma patient transfer system that would provide a platform for reporting immediate and surge burn/trauma bed availability across the U.S. and among NATO partners, and electronically match patient and bed location and match patient acuity with open beds at clinical burn facilities nearby.
“Introducing the HsPro® System”
Athena GTX is proud to present the HsPro® System consisting of multiple portable wellness products that monitor physiological status including Pulse Rate, Heart Rate, Skin Temperature, and ambient environmental conditions during recreational, physical, or training activities.