With support from the US Army Medical Material Development Activity and MTEC, Critical Innovations, LLC has developed the Eye-Aid™ system. The program was received with enthusiasm during extensive military end-user testing and showed efficacy in a live animal model of open globe injury.
The initial management of undifferentiated traumatic ocular injury is a major sight-threatening problem for both civilian and military medicine. Although 10-15% of combat-related trauma involves eye injury, there currently exists limited management options for the far forward medical provider (e.g., medic, physician’s assistant) at Role 1. Difficulties of caring for such patients at this initial role-of-care include lack of provider training, diagnostic equipment, and available treatment modalities. A treatment that can be applied emergently to temporarily tamponade leakage and stabilize the eye, which is also easy to remove upon arrival to specialized ophthalmologic care would be of great benefit to numerous patients.
The patented Eye-Aid™ System for Acute Ocular Injuries provides an ideal far forward solution for temporary eye stabilization following ocular injury. It benefits or, at the very least, does no harm to the wide spectrum of potential eye injuries, while securing, sealing, and preserving ocular tissues. Eye-Aid™ gently spreads topically over eye tissues and can be reapplied multiple times, if necessary. It is also easy to remove upon arrival to specialized ophthalmologic care. Eye-Aid™ is simple and quick to use by far forward personnel, without preparation time or complicated administration processes. The device is also simple to store (e.g., stable in a wide range of environmental extremes) and transport (e.g., small, lightweight, durable), making it particularly well-suited for military use.
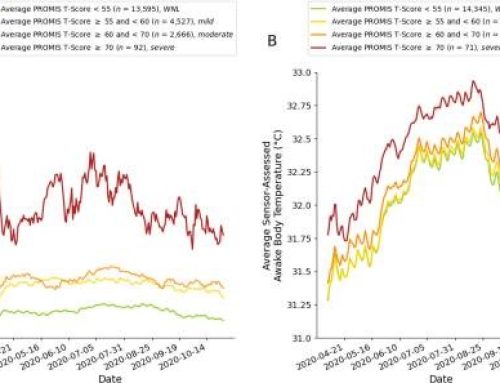
The program met or exceeded all deliverables, was received with enthusiasm during extensive military end-user testing, and delivered very encouraging porcine study data. Preliminary data under this program demonstrated impressive efficacy using Eye-Aid™ in an ACURO-approved proof-of-concept live animal model of open globe injury. In the study, the Eye-Aid™ group demonstrated a 79% eye “save” rate compared to 14% in the control group based on a measure of structural collapse of the anterior chamber, resulting in an impressive Number Needed to Treat of 1.5 for this finding. Results support the hypothesis that the product can provide a protective environment that seals injured ocular tissues until a patient reaches higher level care. Work under this effort also included production of a refined prototype with subsequent refinement for large-scale manufacture.
Critical Innovations was recently selected for award for Phase II SBIR funding through the Defense Health Agency (DHA). This work will focus on optimization of the Eye-Aid™ prototype and next stage preclinical testing in support of a pivotal regulatory submission.
The research project award recipients were selected from the respondents to MTEC’s Request for Project Proposals soliciting medical technological solutions related to MTEC’s Technology Focus Areas (Solicitation #MTEC-20-14-Ocular).
About Critical Innovations, LLC: Critical Innovations is an ISO 13485:2016 certified, 21 CFR§820 Quality System Regulations (QSR) and cGMP compliant, medical research and development company with extensive systems and expertise in the full spectrum of medical device development through regulatory clearance and commercialization. The company focuses on task-shifting evidence-based interventions to earlier echelons of care and more general specialty levels, to simplify management and allow for rapid provision in emergency and combat environments. Based in Los Angeles, the company has worked under more than 40 different Department of Defense awards from all military branches charged with medical technology development (i.e. U.S. Army, Air Force, Navy, and Space Force), including such agencies as the Air Force Medical Support Agency; Army Medical Research and Development Command; Defense Health Agency; and, Office of Naval Research.
Ross Donaldson, MD, MPH, CTropMed, FACEP is President & CEO of Critical Innovations and is triple-boarded in emergency medicine, emergency medical services, and clinical informatics. He has been the principal investigator on over $65 million dollars’ worth of related funding. Additional team members include: Dr. Timothy Fisher, Vice President of Research, a polymer scientist who has an extensive medical device research background and is a former founder of a successfully commercialized medical device startup; and Dr. Jonathan Armstrong, Vice President of Quality Assurance & Regulatory Affairs, a polymer scientist who leads the company’s ISO 13485-certified quality system. The company is working in collaboration with David Tanen, MD, FAAEM, FACMT (USN, Ret.), an emergency physician with multiple combat tours and past residency and research director at the Naval Medical Center in San Diego, and, former Commander Denise Whitfield, MD, FACEP (Former, USN), who has an extensive military out-of-hospital medicine background, previously serving as the Chief of Protective Medicine for the White House and Medical Director for Camp David Emergency Medical Services (EMS). 
Disclaimer: The views expressed in this news article are those of the authors and may not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government.