With support from Combat Casualty Care Research Program and MTEC, Ischemix, Inc. has demonstrated the safety of their lead compound, CMX-2043, in an 80-patient double-blinded, randomized, placebo-controlled Phase 1 clinical trial. The two-part trial tested CMX-2043 in a 40-patient single ascending dose (SAD) and a 40-patient multiple ascending dose (MAD) trial. No serious adverse events were reported, and all treatment-emergent adverse event were mild and self-limiting.
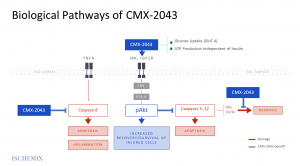
Traumatic Brain Injury (TBI) is a condition that is initiated by the primary injury, but is substantially compounded by secondary injuries, in which the body’s pathophysiological response to the primary insult results in further morbidity. The multi-modal capability of CMX-2043 to activate the PI3k/Akt survival signaling pathways, restore mitochondrial bioenergetics, modulate calcium overload, and reduce reactive oxygen free radical damage could provide the ideal pharmacologic intervention. CMX-2043 is a proprietary new chemical entity based on a scaffold of alpha lipoic acid, an essential cofactor for several mitochondrial energy pathways in human tissues.
At four well-established preclinical laboratories, CMX-2043 has demonstrated safety and efficacy in two models of TBI injury in rodents and in two models of TBI injury in pigs. CMX-2043 has recently generated significant positive neuroimaging and neurobehavioral data in a six-week, multi-dose, randomized, blinded, placebo-controlled porcine TBI study of CMX-2043. Biomarker and histology results are expected later in 2023. Earlier in 2023, CMX-2043 completed a successful single and multiple ascending dose Phase 1 trial. The compound has also previously demonstrated clinical proof of concept in a cardiovascular clinical trial and has shown safety in over 400 patients exposed to the drug in clinical trials for non-TBI indications.
Ischemix intends to undertake a Phase 2 Proof of Concept trial in patients who have suffered a moderate-to-severe TBI. The Company also intends to develop the candidate for treatment of mild TBI (also referred to as concussion).
Research project award recipients were selected from the respondents to MTEC’s Request for Project Proposals soliciting medical technological solutions related to MTEC’s Technology Focus Areas (Solicitation #MTEC-20-15-TBI and MTEC-19-08-MuLTI).
About Ischemix, Inc: Ischemix, Inc is a privately-held pharmaceutical company that is developing novel cytoprotective compounds for serious diseases and conditions. Ischemix, Inc.’s drug candidate, CMX-2043, is a novel, patented, cytoprotective compound in development for the treatment of acute traumatic brain injury (TBI). CMX-2043 will be Phase 2-ready in Q1 2024. Dave DeWahl, the President and CEO of Ischemix been with Ischemix for ten years. Dave’s prior experience includes working as an executive of a start-up biotech company in the DNA repair space, a co-founder of an oncology therapeutics company and as an investment banker working on financings and mergers and acquisitions for health care and life science companies. Jerry Stern has served as the Chief Medical Officer of Ischemix for two years, prior to which he served as a consultant to the Company. Jerry was a Corporate Vice President and global therapeutic area head for Boehringer Ingleheim for more than 20 years and previously was a physician and researcher at NYU Medical Center. Alex Baguisi has been with Ischemix for 20 years and is currently Head of Preclinical Research. Alex was previously in preclinical research at TranXenogen and at Tufts University.
Further reading on the safety and efficacy of CMX-2043 in in vitro and models of other ischemia-reperfusion animal models:
http://www.ischemix.com/pdf/Bioorganic%20and%20Medicial%20Chemistry_2014_505-512.pdf
http://www.ischemix.com/pdf/CMX-2043%20Mechanisms%20of%20Action%20In%20Vitro.pdf
http://www.ischemix.com/pdf/Preclinical%20and%20Clinical%20Safety%20Studies%20of%20CMX-2043.pdf
Disclaimer: The views expressed in this news article are those of the authors and may not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government.