Under a co-funded award from The BrightFocus Foundation and MTEC, the laboratories of W. Brad Hubbard, PhD and Patrick G. Sullivan, PhD in the Spinal Cord and Brain Injury Research Center (SCoBIRC) at the University of Kentucky demonstrated that mitochondrial dysfunction is apparent following repeated, mild blast-induced traumatic brain injury (TBI) and that the dysfunction can be modulated by a novel drug, MP201 (Mitochon Pharmaceuticals, Inc.), to attenuate the disease.
Blast-induced TBI results in ongoing neurological deficits, including psychiatric and cognitive impairments. Low level blast exposure is relatively common in military settings, including training operations. As there are no current treatments approved by the U.S. Food and Drug Administration for mild TBI (mTBI), preclinical studies have sought to characterize the neuropathobiology underlying mild blast TBI (mbTBI) and extensive focus has been on neurovascular disruption and inflammatory outcomes. Recent efforts have been taken to characterize metabolic and mitochondrial outcomes following mbTBI. However, there is a gap in understanding regarding mitochondrial bioenergetic changes and mitochondrial oxidative damage after mbTBI. Further, no study to-date has evaluated a therapeutic approach to targeting mitochondrial dysfunction following mbTBI.
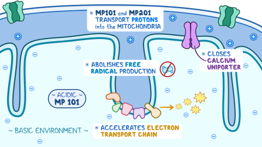
It is well-known that mitochondrial dysfunction underlies many pathological processes and contributes to neurological deficits. To target mitochondrial impairment after TBI, the team has demonstrated that mild mitochondrial uncoupling reduces oxidative stress and is neuroprotective after TBI. A prodrug for mitochondrial uncoupling, MP201 (Mitochon Pharmaceuticals, Inc.), can deliver low, sustained levels of 2,4 dinitrophenol (DNP) that works to reduce calcium burden and lower oxidative stress, which are two hallmarks of TBI pathophysiology and reduce neurological symptoms.
For the next steps in the project, the team now moves forward to demonstrate behavioral efficacy of MP201 in a preclinical model of mbTBI. Further, ongoing studies that enable the filing of an Investigational New Drug application with MP201 are being performed.
This work has been recently published in the Journal of Neurotrauma. The research project award recipients were selected from the respondents to MTEC’s Request for Project Proposals soliciting medical technological solutions related to MTEC’s Technology Focus Areas (Solicitation #MTEC-20-16-mTBI-005).
About University of Kentucky and Mitochon Pharmaceuticals, Inc.:
The laboratories of W. Brad Hubbard, PhD and Patrick G. Sullivan, PhD are both part of the Spinal Cord and Brain Injury Research Center (SCoBIRC) at the University of Kentucky. Our labs are focused on understanding the pathobiology of traumatic brain injury (TBI) and investigating therapeutics in preclinical models that can target critical mechanisms of injury to improve outcomes. Harnessing the power of the mitochondrion after injury via targeted therapeutics can alleviate metabolic and neurological consequences after TBI.
Robert Alonso, Founder & CEO and John G. Geisler, PhD, Founder & Chief Scientific Officer, represent Mitochon Pharmaceuticals. The mission of Mitochon Pharmaceuticals is to bring forward meaningful drugs that work at the root of insidious diseases by direct modulation of the mitochondria’s physiology to attenuate disease progression.
Disclaimer: The views expressed in this news article are those of the authors and may not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government.