ReMDO is a non-profit with a mission to help deliver on the promise of regenerative medicine by promoting progress of biomanufacturing scale-up and automation to make technologies more affordable. ReMDO is excited to be working with MTEC on the Universal Media for the Support and Expansion of Human Cells for Regenerative Medicine Manufacturing. ReMDO has made exciting progress to date with working with two academic teams at Wake Forest Institute for Regenerative Medicine and University of Oregon along with numerous industry collaborators.
One of the roadblocks to the development and manufacturing of regenerative medicine clinical products is the lack of a universal media, as the current paradigm is “one cell type, one media” resulting in numerous media formulations requiring evaluation and approval by the FDA. The overall goal of the ReMDO Media Program is to develop a set of unifying culture medias, both serum free and xenofree with compatible bio-coatings for the expansion of numerous clinically relevant human primary cells derived from each germ layer, and to develop standard operating procedures (SOPs) for performance testing and media use.
Some of the noteworthy accomplishments include:
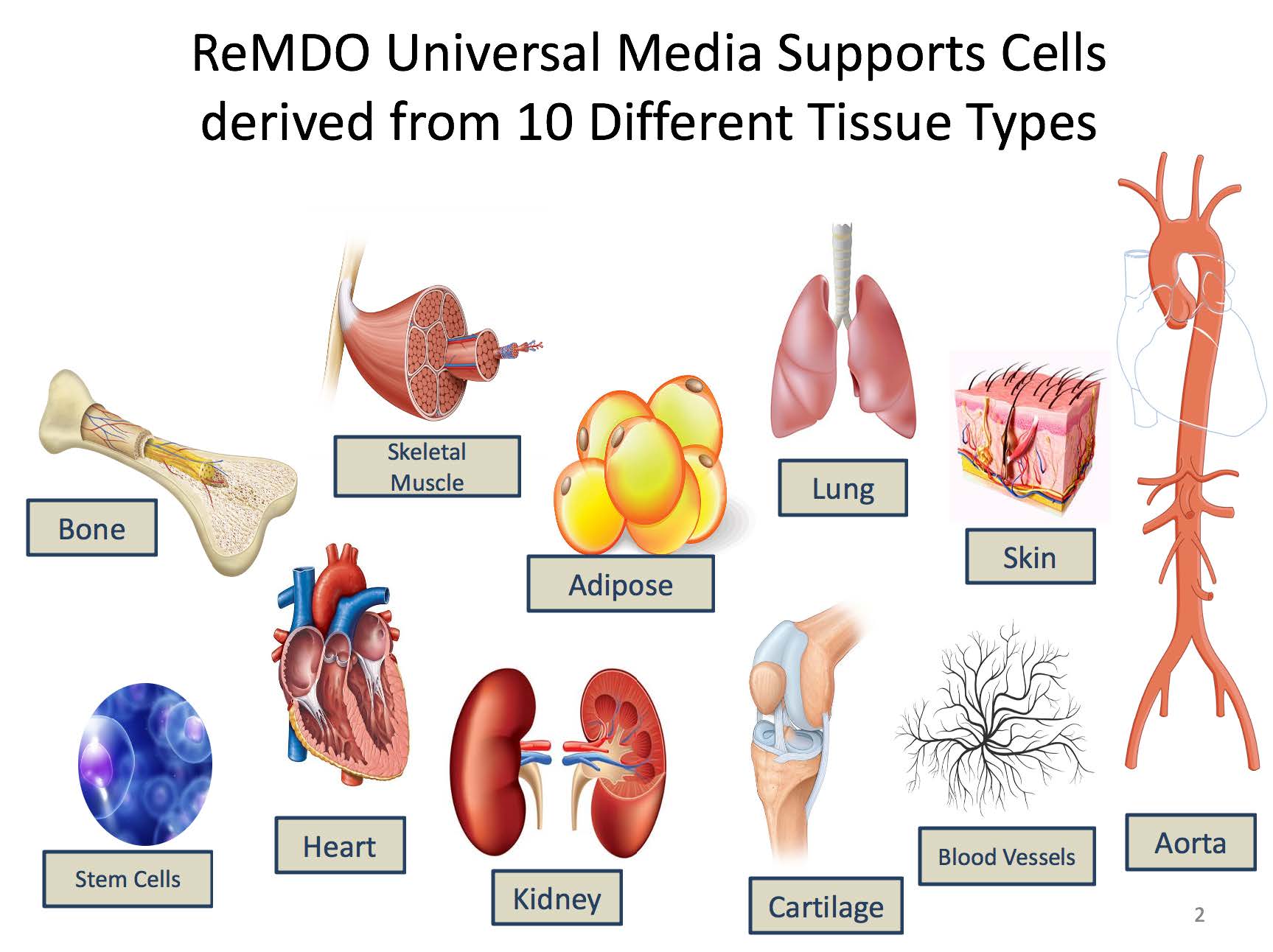
- Development of 2 Universal medias that are standardized serum free xenofree media and have been shown to support clinically produced cell types across all three germ layers. (more than 20 primary human cell types tested).
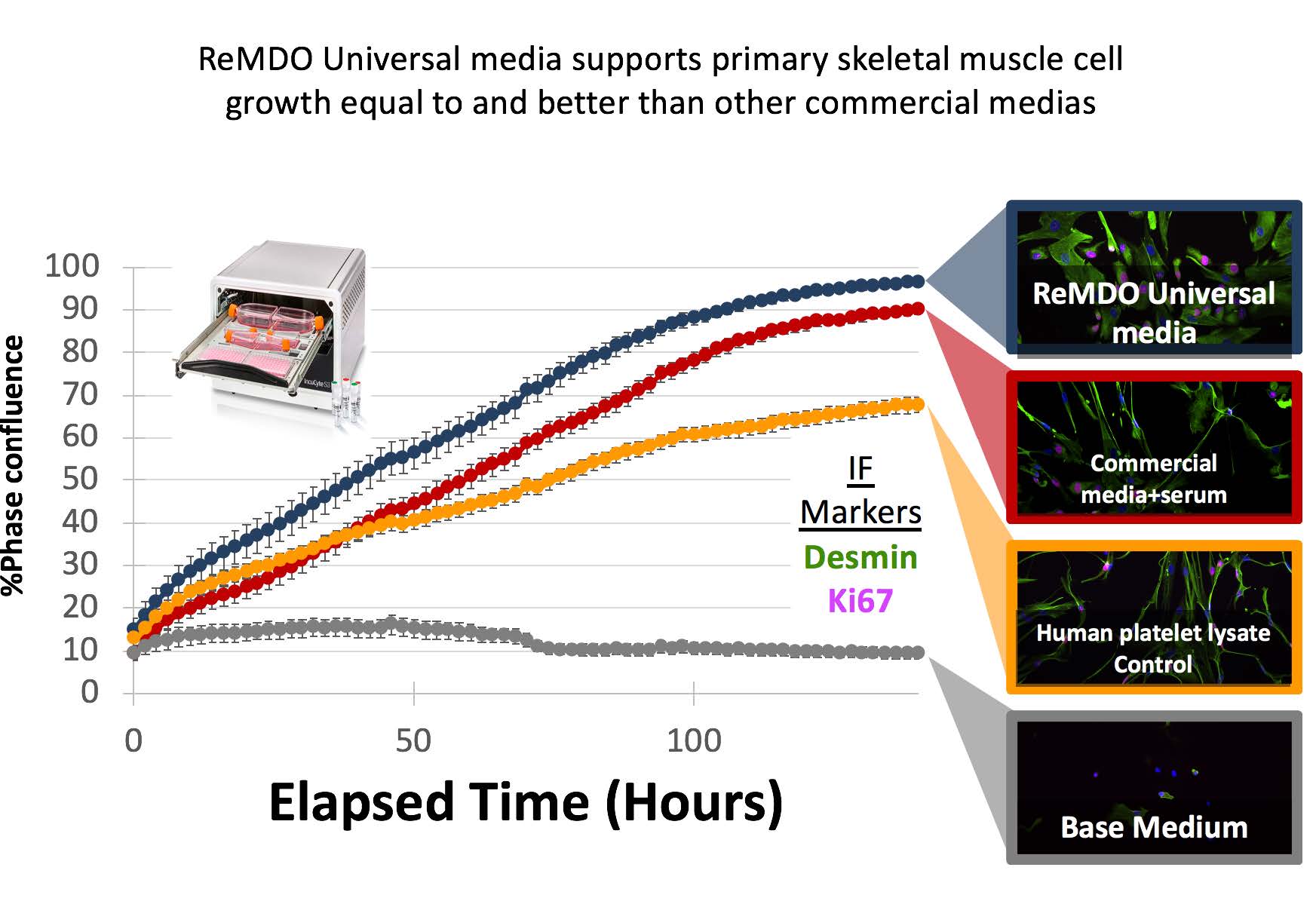
- Develop standardized “cell performance sheets”- a set of standardized growth curves, morphology panels and cell validation metrics to set expectations of cells grown in the universal medias.
- Collaborated with biomedical industry leaders to develop standardized immunophenotypic panels for characterizing cells using flow cytometry and immunocytochemistry techniques.
- Demonstrated a comparable clinical scale expansion of bone marrow mesenchymal stems (BM-MSC) using ReMDO universal medium.
- Performed an independent secondary site validation of universal media performance using mesoderm and ectoderm derived cell types.
- Successfully worked with Industry cost share partners to develop supplement packs comprised of FDA approved reagents.
- Established documentation for over thirteen different research related processes to develop standard procedures and validation strategies.
- Successfully worked with an industry collaborator to manufacture 20L of our final chemically-defined, xeno- free medium completely off of a cost share arrangement.
- Biocoatings and cell growth boosters are being developed to reduce costs and improve cell growth in the clinical manufacturing environment. We are grateful for the opportunity to work with MTEC to assist in moving the needle forward for the field of regenerative medicine by developing manufacturing solutions.