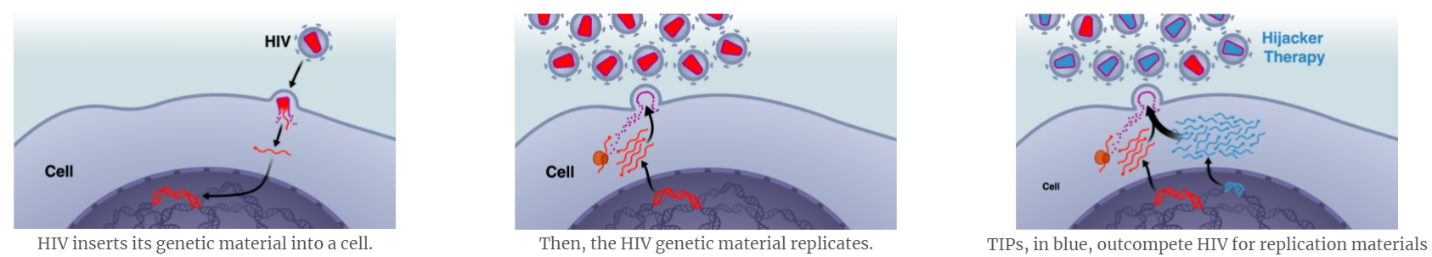
Background: Deployment barriers and the genetic diversity (mutation) of HIV present major obstacles to reducing HIV prevalence using conventional drug therapies and potential vaccines; antiretroviral therapies require lifelong administration. The Weinberger lab at the Gladstone Institutes at the University of California, San Francisco has developed a novel therapeutic paradigm to reduce HIV prevalence and incidence. The approach, named Therapeutic Interfering Particles (TIPs), are engineered, synthetic deletion variants that outcompete HIV for essential proteins within infected cells t substantially reduce HIV burden in vivo to halt viral transmission and disease progression.
Preliminary results: Recently, the Weinberger lab developed prototype TIPs against HIV-1 and demonstrated safety, efficacy, and broad-spectrum anti-HIV activity. With MTEC funding, the team recently demonstrated that TIP safety and efficacy; they showed that TIPs reduce HIV titers by over 100 fold in humanized mice and protect CD4+ T cells from HIV infection in these mice. The team further validated that TIPs are safe (do not replicate in the absence of HIV), and can mobilize to protect bystander cells in the presence of HIV co-infection. The team also demonstrated that TIPs have an exceptionally high genetic barrier to resistance. The FDA provided initial approval for Phase-I clinical trials on these data and the concept was highlighted in a recent TED Talk.
Ongoing studies: The success of the prototype TIPs in humanized mice represents a significant step towards translation. However, to move forward to clinical trials the results must be extended by assessing dose, time of treatment, biodistribution, genotoxicity, and transmission potential of TIPs in humanized mice models and non-human primates (NHPs). SIV-infected Rhesus macaques (RM) are the benchmark animal model for testing safety and efficacy of experimental HIV interventions as they establish a setpoint viral load similar to humans, and develop an AIDS-like disease. The proposed studies are to test TIP safety (genotoxicity and immunogenicity) and efficacy in the NHP RM model.
Impact: Epidemiological analyses project that TIPs could significantly outperform existing and proposed interventions (e.g. antiviral therapies and vaccines), stably lower HIV/AIDS prevalence below 1% within 20 years. Strikingly, TIPs could also complement current therapies on the population level.
TIPs promise key innovations over current antivirals and potential vaccines including the ability to be resistance-proof, single-administration, and population-wide control interventions.
Team: Dr. Leor Weinberger’s group first proposed the TIP concept over 20 years ago and discovered the first TIP in 2018. The lab is dedicated to developing TIPs as single-administration population-scale HIV therapy for difficult-to-reach populations (e.g., injection drug users) and resource-poor settings (e.g., sub-Saharan Africa).
Additional Links:
- TIPs Executive Summary – overview of TIPs concept
- TIPs YouTube Talk – talk at UCSF explaining TIPs with a child’s toy
- Piggyback Virus Could Curb HIV Pandemic. WIRED. March 21, 2011