With support from USAMRDC’s Military Infectious Diseases Research Program (MIDRP), VxBiosciences and the Gladstone Institutes at the University of California, San Francisco are developing and testing Therapeutic Interfering Particles (TIPs) to reduce population-level COVID-19 transmission. TIPs, which are a novel therapeutic paradigm that have the potential to reduce viral transmission and disease, were originally developed at the Weinberger Lab at the Gladstone Institutes. The TIP technology uses engineered-deletion mutants, lacking all pathogen genes, that compete for essential viral pathogens proteins intracellularly and thus suppress wild-type virus to reduce viral burden in infected tissues. By reducing viral loads, it was hypothesized that TIPs engineered against SARS-CoV-2 would significantly reduce COVID-19 pathogenesis and disease transmission. For this project, VxBiosciences Inc. partnered with the Gladstone Institutes to engineer nanoparticle TIPs optimized for SARS-Cov-2 and accelerate TIP technology through regulatory and commercialization pathways.
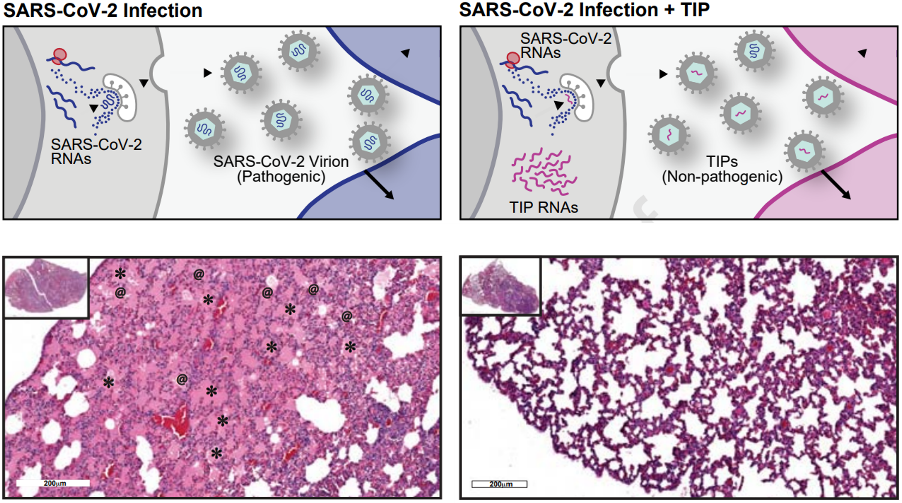
Recently, the team published a paper in Cell that comprehensively describes the biological mechanism of action of TIPs and demonstrated efficacy against SARS-CoV-2 in the leading animal model of infection (Syrian hamsters). In this paper, the team showed that: 1) TIPs inhibit SARS-CoV-2 in human lung organoids, 2) SARS-CoV-2 does not evolve to escape TIPs, 3) a single (intranasal) administration of TIP nanoparticles significantly reduced viral load in the lungs of hamsters, and 4) TIPs suppress inflammation and severe disease in hamsters when administered either pre- or post-infection. Together, these data indicate that if successfully translated to the clinic, TIPs could represent a class of single-administration antivirals with a high genetic barrier to the evolution of resistance.
Future plans involve finalization of nanoparticle formulation as a vehicle for delivery, determination of the appropriate commercial delivery device, IND submission to the FDA, and initiation of human clinical trials. Cryopreservation and characterization studies are also ongoing to enable field deployment.
The target market for TIPs is widely applicable to military and civilian populations given that TIPs are single-administration interventions that could be utilized in a pre-exposure prophylactic or therapeutic setting, co-evolve with targeted wild-type viruses and may be a powerful tool against a variety of emerging infectious-disease threats. This project was funded through MTEC’s Request for Project Proposals for treatments with the potential application to prevent COVID-19 infection (Solicitation #20-09-COVID-19_Treatment_MIDRP).