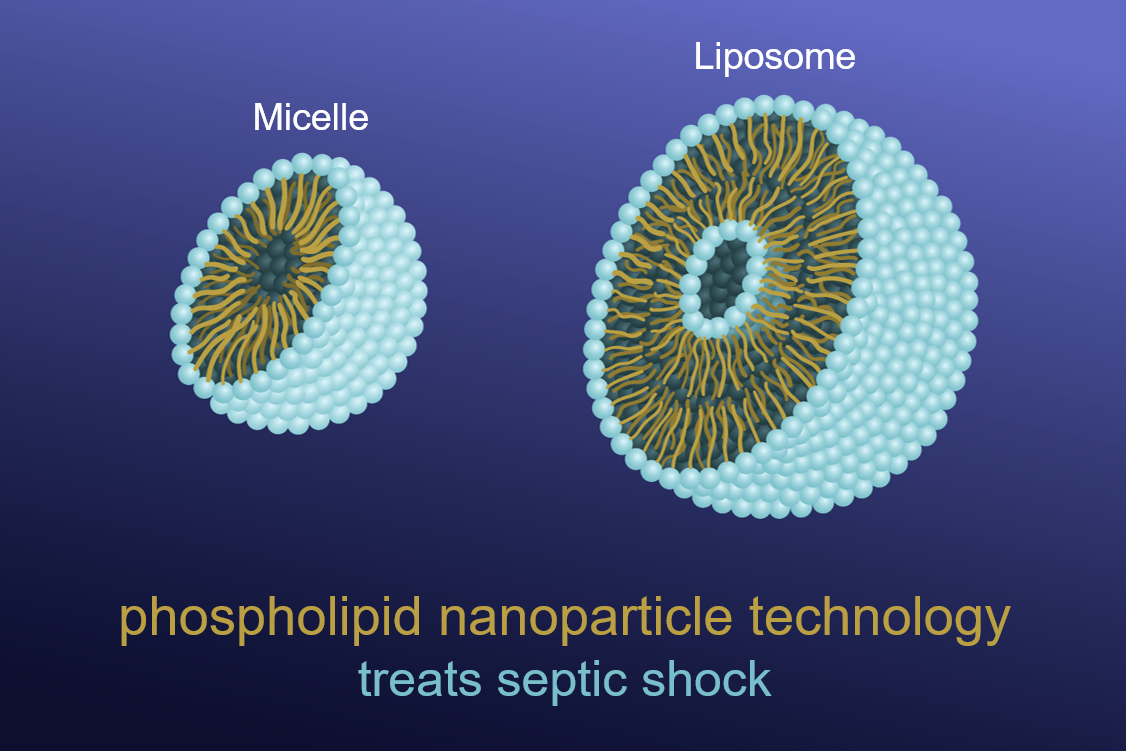
Vivacelle Bio was recently cleared by the FDA to proceed with its Phase IIa clinical trial for evaluation of the safety and efficacy of VBI-S, an intravenously injected cardiovascular support fluid containing phospholipid nano-micelles and liposomes to treat septic shock. Septic shock is a potentially fatal condition that results from the body’s systemic response to infection and leads to critically low blood pressure and organ dysfunction. Septic shock is prevalent in 70% of COVID-19 fatalities and is also a major cause of mortality and morbidity in combat casualties. According to the WHO, septic shock is at least partially responsible for 10 million deaths per year worldwide. This novel treatment is likely to be life-saving, where it is expected to elevate blood pressure to survivable levels and improve oxygenation in patients with septic shock, even when standard treatment has failed.
With support from the Naval Medical Research Center (NMRC) and MTEC, Vivacelle Bio has completed preclinical studies indicating that the beneficial action of VBI-S occurs through its unique ability to redistribute nitric oxide, a gas that is overproduced in septic shock and causes potentially fatal hypovolemia, hypotension and organ damage. The overproduced nitric oxide (hydrophobic in nature) preferentially localizes (binds) to the hydrophobic nanoparticles which reduces the bioavailability of nitric oxide without stopping its production. Another favorable aspect of this nitric oxide modulation is that it is readily reversible; thus, the nitric oxide can be released where it is needed in the body. The safe redistribution of nitric oxide is a fundamentally new approach with potential applications to many disease processes in which nitric oxide is involved.
Cuthbert Simpkins, M.D., a trauma surgeon and inventor of the phospholipid nanoparticle technology at Vivacelle Bio stated, “Finally, we have what I and others have been seeking for all of this time, a way to save lives and to reduce suffering of patients and their loved ones.” Recruiting for the Phase IIa clinical trial is currently underway. Details about the clinical trial, which is titled, VBI-S for the Treatment of Hypotension in Hypovolemic Septic Shock Patients, can be found online at ClinicalTrials.gov. For more information, visit VivacelleBio.com and watch an interview with Vivacelle Bio CEO, Dr. Harven DeShield.
This project was funded through MTEC’s Advanced Biomedical Product Development for Naval Operations Request for Proposals (Solicitation #20-02-NavyMulti).
About Vivacelle Bio:
Vivacelle Bio, Inc. is an advanced clinical stage biopharmaceutical company that develops improved treatments for shock due to infection, blood loss, and other critical illnesses. Vivacelle Bio has a pipeline of products based upon its transformational phospholipid nanotechnology.