Traumatic acute subdural hematomas, which are clots of blood that develop between the brain surface and dura mater, are life-threatening injuries associated with traumatic brain injuries that can cause elevated intracranial pressure (ICP > 20 mmHg). Such injuries require immediate diagnosis and treatment. However, far-forward medical personnel lack portable, easy-to-use devices for in-field diagnosis of traumatic brain injury (TBI). The deployment of artificial intelligence (AI)-enhanced point-of-care ultrasound (POCUS) systems have the potential to dramatically impact the initial care and triage of wounded warfighters.
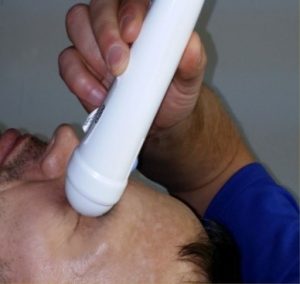 A major barrier to widespread adoption of POCUS systems is the required expertise in ultrasound acquisition and interpretation. In response, Kitware is designing an automated and easy-to-use software system to detect life-threatening elevated intracranial pressure that only requires a medic, with no formal ultrasound training, to place the probe the patient’s eye and rock the probe back and forth. Ultrasound of the eye allows visualization of the optic nerve, which is a direct extension of the brain meninges, and thus allows noninvasive estimation of intracranial pressure. Kitware’s software system utilizes a deep learning AI system that automatically identifies the video frames that contain the eye and optic nerve, and produces an estimate of optic nerve sheath diameter that increases in reliability over each sweep of the eye. The algorithm incorporates an innovative statistical model of patient-specific correlations (i.e., age, ocular orb size, and BMI) to more accurately determine changes in a patient’s optic nerve sheath diameter, which is indicative of an increase in intracranial pressure. Thus, this technique can determine when urgent, life-saving treatment is needed for TBI patients.
A major barrier to widespread adoption of POCUS systems is the required expertise in ultrasound acquisition and interpretation. In response, Kitware is designing an automated and easy-to-use software system to detect life-threatening elevated intracranial pressure that only requires a medic, with no formal ultrasound training, to place the probe the patient’s eye and rock the probe back and forth. Ultrasound of the eye allows visualization of the optic nerve, which is a direct extension of the brain meninges, and thus allows noninvasive estimation of intracranial pressure. Kitware’s software system utilizes a deep learning AI system that automatically identifies the video frames that contain the eye and optic nerve, and produces an estimate of optic nerve sheath diameter that increases in reliability over each sweep of the eye. The algorithm incorporates an innovative statistical model of patient-specific correlations (i.e., age, ocular orb size, and BMI) to more accurately determine changes in a patient’s optic nerve sheath diameter, which is indicative of an increase in intracranial pressure. Thus, this technique can determine when urgent, life-saving treatment is needed for TBI patients.
Furthermore, Kitware’s software is compatible with a wide range of commercial ultrasound probes. Over the course of this project, Kitware has developed a unified application programming interface (API) to work with Clarius, Butterfly, Sonivate, SonoQue, and Interson ultrasound probes. As part of Kitware’s commitment to open source and open science, they have released this work to the community as an open source software package called ITK POCUS (the ITK POCUS Python package can be found here). Kitware recently demonstrated the capabilities of ITK POCUS at the Advances in Simplifying Medical Ultrasound (ASMUS) workshop at the International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI).
Kitware is in the midst of a human study (with healthy volunteers and emergency TBI patients) to test the real-time AI algorithm with the previously listed ultrasound probes and determine impact on the performance of the AI. As part of this human study, Kitware has also developed an improved eye phantom which is housed in a head mannequin to more closely mimic human anatomy, calibrate the commercial ultrasound probes, and ensure all measurements are consistent and accurate. As Kitware continues its human study, which will conclude in 2022, they are preparing to initiate two additional user studies. One user study will be performed with unskilled operators and the other with clinicians and will examine each probe’s suitability (in terms of video quality and ergonomics) for measuring optic nerve sheath diameter.

The predicted optical nerve sheath diameter (ONSD) increases in reliability with each sweep of the eye.
This project was funded through MTEC’s Request for Project Proposals for life-saving trauma innovations (Solicitation #19-08-MuLTI), sponsored by the U.S. Army Medical Research & Development Command (USAMRDC) Combat Casualty Care Research Program (CCCRP). This project is led by Principal Investigator, Dr. Stephen Aylward, Senior Director of Strategic Initiatives at Kitware. This project is supported by Project and Clinical Leads, Dr. Brad Moore of Kitware and Dr. Sean Montgomery of Duke University Medical Center, respectively. Dr. Montgomery, in the midst of the COVID-19 pandemic and his own responsibilities in Duke’s Trauma Surgery Unit, has managed an early start to the project’s human study. Research and development at Kitware is led by R&D Engineers, Tom Osika and Tim Thirion, who have brought valuable expertise in deep learning AI and commercial point-of-care ultrasound applications, respectively, to the project.
About Kitware:
The Medical Computing group at Kitware is a team of deep learning AI specialists, software engineers, and imaging experts who provide collaborative research, development, and technology integration services for research centers, the U.S. government, and companies working in the medical and biomedical business sectors. They have a long history leading and contributing to open source platforms such as the Insight Toolkit (ITK), vtk.js, and 3D Slicer, that serve as the foundation of many medical visualization and data processing applications. They work with their commercial customers to streamline their internal software and processes by investigating new algorithms or methodologies that upgrade existing commercial offerings or develop new products. Alone or as part of partnerships, Kitware generates results for research publications, develops prototypes for raising venture capital, and conducts first-in-human trials.