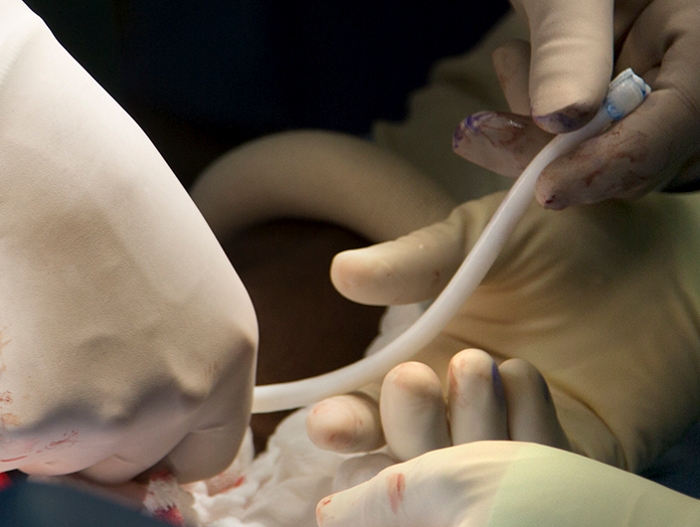
Humacyte, a regenerative medical technology company based in Durham, North Carolina is pioneering the development and manufacturing of off-the-shelf, universally implantable, bioengineered tissues. Its technology platform can generate bioengineered, human acellular vessels, or HAVs, which can regenerate and have the potential to become the patient’s own living tissue. The HAVs are designed to be easily implanted into any patient without inducing a foreign body response or leading to immune rejection.
Support and funding from MTEC has aided in the development and validation of Humacyte’s manufacturing process in order to bring the HAV to patients in need. Recently, these patients have included veterans at Walter Reed National Military Medical Center.
In May of 2019, Humacyte received a special request from the US military to assist with a surgical bypass procedure by providing the HAV product for a retired military veteran. Supported by an expanded access approval by FDA, the patient received a bioengineered blood vessel created by the Humacyte manufacturing process to restore blood circulation to the patient’s leg. “Having been involved with the research and development of this product as part of our requirements-driven combat casualty care research program since around 2011, it has been especially rewarding to be able to implant the HAV in several patients at Walter Reed over the past couple of years. Our ability to use this novel technology has required close collaboration with partners at the FDA, as well as great support from the company,” said Todd E. Rasmussen, MD, FACS and Colonel USAF MC, F. Edward Hébert School of Medicine, Uniformed Services University. “We’ve now used the HAV in 4 patients at Walter Reed under FDA expanded access conditions and consulted in its use in one patient at the University of Virginia Health System in Charlottesville. At this point we are excited with the results in these cases and see the HAV as a potentially transformative way to treat combat injured with vascular trauma and select numbers of military beneficiaries with vascular disease.”