With support from the Defense Health Agency, J-9 Research and Development (DHA R&D) and USAMRMC, the RegenMed Development Organization (ReMDO) is developing a universal standardized bioreactor platform for the maturation of regenerative medicine clinical products. This system will be scalable across the clinically relevant range and configurable for clinically relevant construct geometries.
Tissue-engineered products are often generated by applying cells to an appropriately shaped biomaterial scaffold. Because these products are “living”, specialized bioreactors are required to provide optimal conditions typically supplied by the host circulatory system and surrounding tissues. Bioreactors play a critical role in preconditioning cells and facilitate growth and maturation within the scaffold. Currently, the regenerative medicine industry designs and fabricates custom bioreactors for each tissue-specific product. The cost and time associated with developing and customizing these individually built bioreactors are very high. This project aims to design and fabricate a standardized, scalable, modular, and configurable bioreactor platform to address this limitation. Fulfilling this need can make regenerative medicine technologies more affordable and widespread and easy access to military personnel and civilian populations. Regenerative medicine technologies can offer transformative cures to military service men and women that cover replacing, repairing, and regenerating any tissue/organ system in the body and offer targeted solutions for personalized medicine.
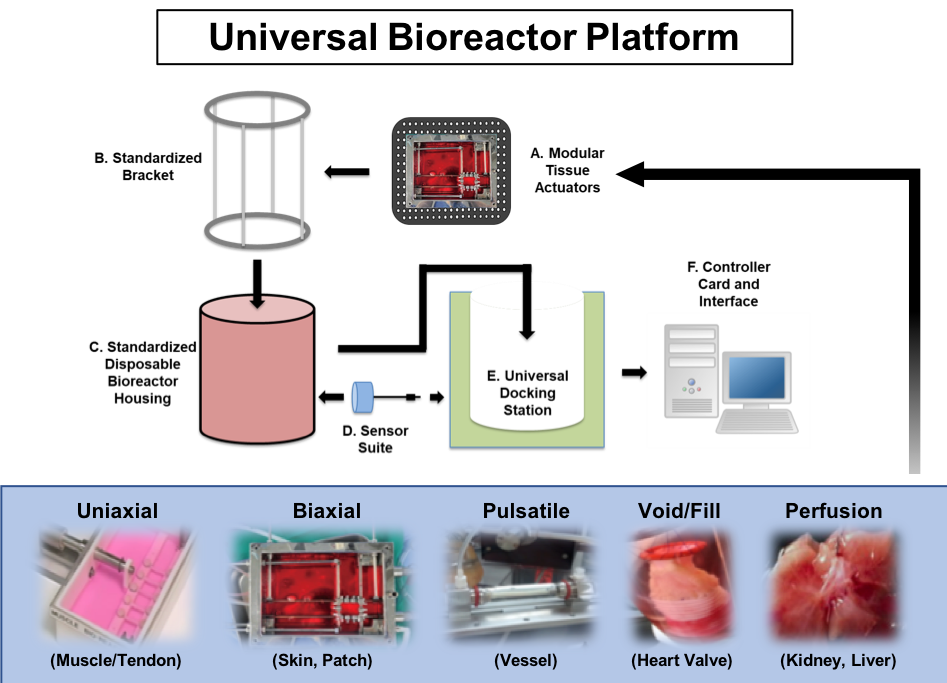
Our technology incorporates all essential bioreactor features into a modular “plug-and-play” universal bioreactor platform that accommodates a wide range of tissue and organ types. We have standardized each component with biocompatible materials following the regulatory-compliant release criteria. Our efforts are directed toward mass manufacturing the standardized bioreactor platform for the regenerative medicine industry to fill the gap in translational processes. The standardized universal bioreactor platform will provide the regenerative medicine industry with a critical tool at reduced manufacturing costs, which will significantly accelerate the clinical translation.
ReMDO has made significant progress toward developing its standardized universal bioreactor platform. The following lists several key accomplishments:
Established criteria for selecting materials for manufacturing specific components of the bioreactor based on the feedback from our industry collaborators through a user requirements questionnaire.
Designed and constructed the uniaxial and biaxial bioreactor modules with integrated sensors to monitor environmental conditions and tissue function. These bioreactor modules were successfully validated using living cells and tissues. The uniaxial module is suitable for tissues with unidirectional structural orientation and function, such as skeletal muscle. The biaxial module is used for flat tissues such as skin and fascia.
Following the manufacturing of the structural tissue bioreactor module prototypes, ReMDO has developed bioreactor modules with complex functions, including tubular (blood vessel), hollow (bladder), and solid (kidney, liver) tissues and organs. The design and fabricated prototypes of each module were validated with living cells and tissues.
As ReMDO continues to develop its bioreactor technology, ongoing efforts are focused on developing the universal bioreactor docking station that could accommodate the interchangeable bioreactor modules. Further steps include completing the standardization of bioreactor housing, monitoring sensors/controller unit integration, system validation, and optimization.
This project was funded through MTEC’s Request for Project Proposals for the development of scalable, production-ready, commercial prototypes for cell, tissue, or organ bioengineering technologies (Solicitation #MTEC-19-07-Biomfg).
About RegenMed Development Organization:
RegenMed Development Organization or ReMDO is a 501c3 non-profit organization seeking to accelerate regenerative medicine technologies by addressing manufacturing challenges. ReMDO has many initiatives that lead to fulfilling this mission and help to bring regenerative medicine technologies to the patient and have these technologies become the next standard of care for medicine.
We have made significant progress with this project, which would have been impossible without the dedicated team of individuals and industry partners. These include talented scientists with diverse backgrounds, research and project managers, and industry partners that share their established technologies.
The following is a list of the bioreactor team:
ReMDO: Joshua Hunsberger, Kelly Burkett, Gary Green
WFIRM: Adit Mehta, Po-Feng Lee, Young Min Ju, Eric Renteria, Drake Sime, Diana Lim, Metin Gurcan, Thomas Shupe, Tracy Criswell, Ji Hyun Kim, Frank Marini, Shay Soker, James Yoo
Industry Partners: PHC Group, Scientific Bioprocessing Inc., AlloSource, Histogen, Millipore Sigma, Qiagen, Rooster Bio, Blood Cell Storage Inc.





