With the support from the USAMRDC’s Military Infectious Diseases Research Program (MIDRP), Inhalon Biopharma, in collaboration with Celltrion, is developing clinical-stage immunotherapies with an inhaled muco-trapping antibody platform to prevent and treat acute respiratory infections, such as SARS-CoV-2 (SARS), respiratory syncytial virus (RSV), influenza, human metapneumovirus (hMPV), and Middle East Respiratory Syndrome (MERS).
Each year millions of people develop respiratory infections which, in many cases, lead to hospitalization and death. COVID-19 alone has caused over one million deaths in the US since 2020. From the unavailability of treatments in the beginning of the outbreak to the viral variant escape from vaccines and therapeutics, the pandemic has demonstrated the urgent need for solutions that can be more rapidly developed and deployed. This need is reinforced when risk factors and transmissibility are initially unknown in an emerging infectious disease situation. Our goal is to deliver a readily available solution that is safe, effective, and convenient for self-administration in any civilian or military setting.
Efforts towards that goal have led to the development of an inhaled muco-trapping formulation (IN-006) of Celltrion’s IV-delivered antibody for treating COVID-19. Inhalon has completed a Phase 1 clinical study in healthy volunteers to demonstrate the safety and tolerability of inhaled delivery of IN-006. Two pre-publications have resulted from this work, one covering the preclinical, IND-enabling studies and one describing the results of the Phase 1. The initial pharmacokinetic (PK) results from the Phase 1 were encouraging in that drug was retained in the lungs for up to five days after a single dose, and there was a significant amount of drug that made it into the circulatory system. These results open the door to potentially treating patients with a single inhaled dose.
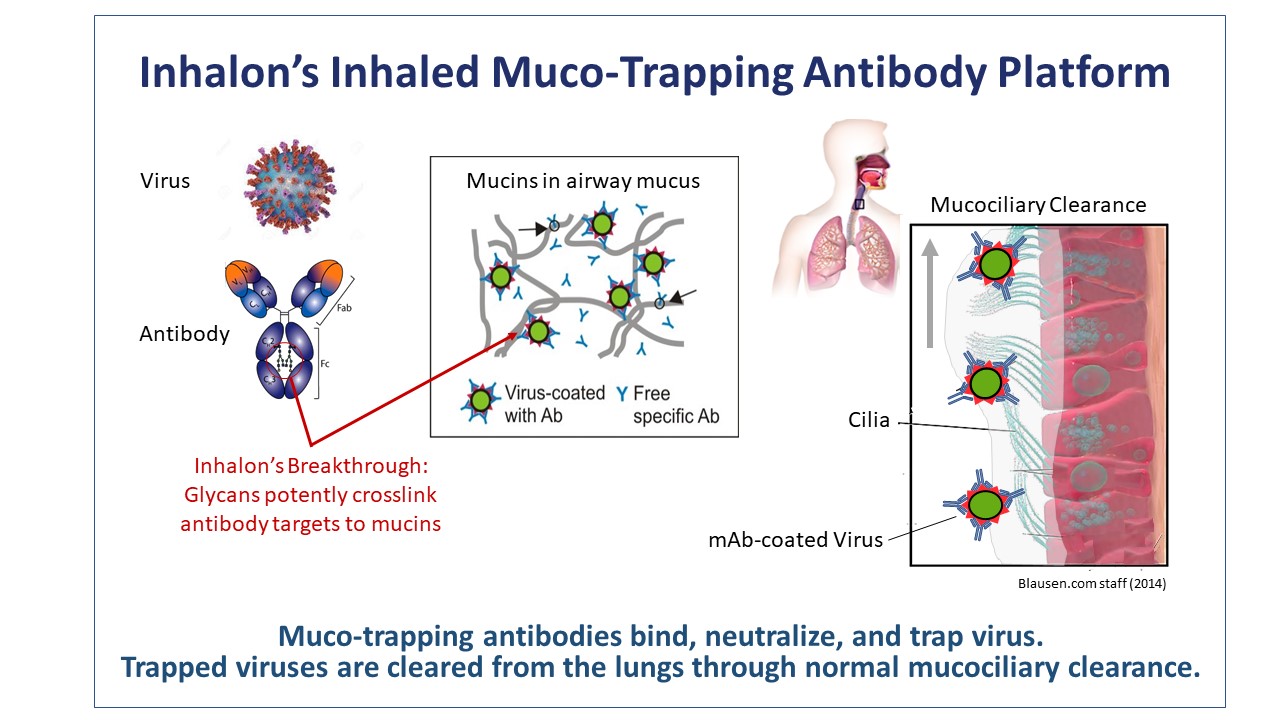
Inhalon’s breakthrough platform enables inhaled “muco-trapping” antibodies to trap respiratory pathogens by crosslinking them to the mucins present in airway mucus. These antibodies stop the infection, and potentially transmission, by neutralizing and trapping the virus in mucus. Once trapped, the pathogens are then eliminated through normal mucus clearance every 30 to 60 minutes into the digestive tract and destroyed by stomach acid. Our patented platform achieves the optimum binding affinities whereby antibody-pathogen-mucin complexes are rapidly cleared from the respiratory tract, while unbound antibodies are retained in the airways for a prolonged duration of effect. In multiple in vivo studies, our platform has demonstrated a ≥ 4-log reduction in viral load to undetectable or background levels. Inhalation of muco-trapping antibodies is the only therapeutic modality that can physically clear pathogens from infected lungs and potentially limit transmission to other individuals.
Inhalon is currently preparing for an inhaled Phase 1b pharmacokinetics study with IN-006. This study is designed to help refine the dosing regimen to treat COVID-19 as well as for other respiratory infections, such as pandemic influenza.
This project was funded through MTEC’s Request for Project Proposals for treatment with potential application to prevent COVID-19 infection (Solicitation #20-09-COVID-19_Treatment_MIDRP).
Meet the Minds at Inhalon
John Whelan is the chief executive officer and has over two decades of experience leading private and public biotech/pharma companies.
Sam Lai, Ph.D. is the chief scientific officer and founder and pioneered Inhalon’s platform technologies as well as the foundational technologies to several FDA-approved therapies. Dr. Lai is also a professor at The University of North Carolina at Chapel Hill.
Jeff Hutchins, Ph.D. serves as the chief development officer and has over four decades of experience in monoclonal antibody and antiviral therapy development in both biotech and large pharma environments.
Tom Moench, M.D., vice president of clinical development, is an infectious disease expert from Johns Hopkins with over two decades of phase 1-3 clinical development experience.





