Extracellular Matrix (ECM) Hydrogels for Regenerative Medicine Applications
Published on April 4, 2025
Hydrogels are highly hydrated materials and can be derived from components of the extracellular matrix to facilitate immune activity and act as a scaffold for new cell growth. The Badylak Lab at the University of Pittsburgh has created lightweight, portable and temperature-tolerant hydrogels that can readily be used at the point of care in a variety of military operations, including rapid clotting for treatment of traumatic injuries on the battlefield.
Project Highlight
With support from the Defense Health Agency J‐9 Research and Development (DHA R&D) Directorate, the Steve Badylak Laboratoryat the McGowan Institute for Regenerative Medicineat the University of Pittsburgh is developing alightweight, portable and temperature-tolerant hydrogel that can be used at the point-of-care in a wide range of military operations, including rapid clotting in low pressure bleeds. In fact, this technology has led to the establishment of a new company – ECM Therapeutics.This project was funded through MTEC’s Request for Project Proposals for developing production‐ready prototypesof regenerative medicine based therapies (Solicitation #19-07-Biomfg).
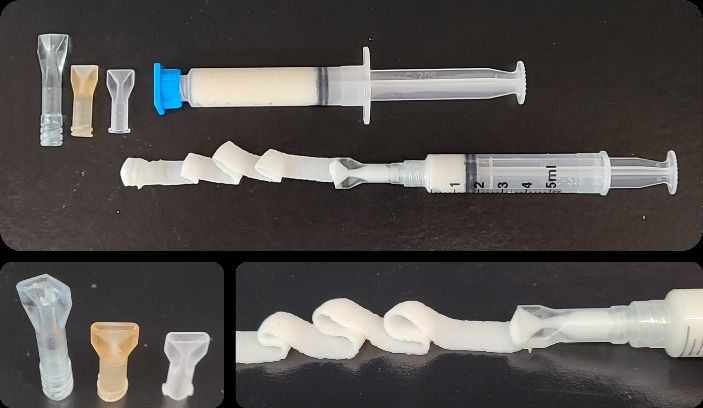
Hydrogels, which are highly hydrated polymer materials, composed of extracellular matrix (ECM) have been shown to improve immunomodulatory activity, recruit endogenous stem/progenitor cells to the anatomic site of placement, and promote the replacement of injured or missing tissue.However, clinical translation of ECM hydrogels, including for battlefield point-of-care, depends on development of large-scale manufacturing techniques and a resulting product that is readily sterilized, portable, has a robust shelf life at a range of temperatures, and can meet industry standards for safety and efficacy. Current commercially-available surgical hemostatsoften require restrictive storage conditions and/or lengthy preparation procedures that are difficult for untrained personnel. In contrast, the point-of-care ECM hydrogel presented here is a stable, off-the-shelf formulation that is ready-to-use with little training or preparation; and can be easily administered to initiate rapid and sustained hemostasis.
Since the start of the MTEC award, the Badylak Lab has:
- developed and validated a cost-effective, proprietary cGMP manufacturing strategy for their point-of-care ECM hydrogel,
- identified FDA-approved methods for packaging and sterilization of the ECM hydrogel,
- designed a wide-mouth, luer-locking applicator tip which allows for more precise application of the ECM hydrogel to the sites of bleeding, and
- demonstrated the robust invitroand in vivo hemostatic properties of the ECM hydrogel.
Moreover, using non-MTEC funds, the Badylak lab completed preclinical liver laceration studies which showed that the ECM hydrogel can initiate rapid clot formation in a high volume, low pressure bleed. Importantly, the hydrogel can be easily removed after cessation of bleeding. Additionally, the hydrogel formulation has other potential therapeutic applications beyond routine surgical procedures including its use in acute traumatic soft tissue injuries, bulk tumor resection, and whole organ hemorrhage.
Withthe ability to cGMP manufacture large-scale clinical batches of ECM hydrogel, the next step in this project is to advance to Phase I clinical trials. This effort includes meeting FDA regulatory requirements for Class III medical devices by conducting animal performance testing and formal ISO 10993 testing, followed by clinical safety and efficacy trials in a 10-patient study.
About the Badylak Laboratory at the University of Pittsburgh:
The Steve Badylak Laboratory at the University of Pittsburgh has significant expertise inECMstructure and function. More than 70 patented ECM-based technologies originating in this laboratory have resulted in the treatment of more than 12 million patients. The emphasis upon clinical translation has resulted in the establishment of several companies including ECM Therapeutics – the industry partner that played a major role in developing a method to upscale manufacture an ECM hydrogel.Special thanks to Dr. George Hussey who conducted the majority of benchtop and in vivo studies in completion of this work.
Featured Member

University of Pittsburgh
The Center for Military Medicine Research (CMMR) at the University of Pittsburgh is dedicated to advancing medical research for wounded service members and their families. The center focuses on trauma, emergency, and critical care, leveraging the university's strengths in medical and engineering fields, and collaborating with Carnegie Mellon University's robotics and AI expertise. CMMR aims to enhance trauma care for warfighters and civilians, contributing to the defense innovation economy.
Visit Profile