Electroceutical Technology Against Bacterial Drug Resistance
Published on April 04, 2025
Bacterial drug resistance remains a significant threat to Service Members. In response, Indiana University has performed preclinical studies to establish the electroceutical based EDT technology. This technology is a flexible fabric-based disposable dressing that generates a low level of electricity in the wound milieu causing injury to the bacteria and making it more susceptible for adjunct therapies.
Project Highlight
Bacterial drug resistance remains a significant threat to Service Members. Over the past century, antibiotics have played a crucial role in healthcare, including helping achieve many major advancements in military medicine. However, with the ongoing antibiotic resistance crisis, the military seeks novel platforms for the delivery of wound care anti-infectives at the point-of-injury, with a special emphasis on treatments that are integrated intodressings and bandages. In response, with funding support from an MTEC award, Dr. Sashwati Roy at Indiana University has performed preclinical studies to determine efficacy of electroceutical dressing technologies (EDT) in mitigating wound biofilm infection.
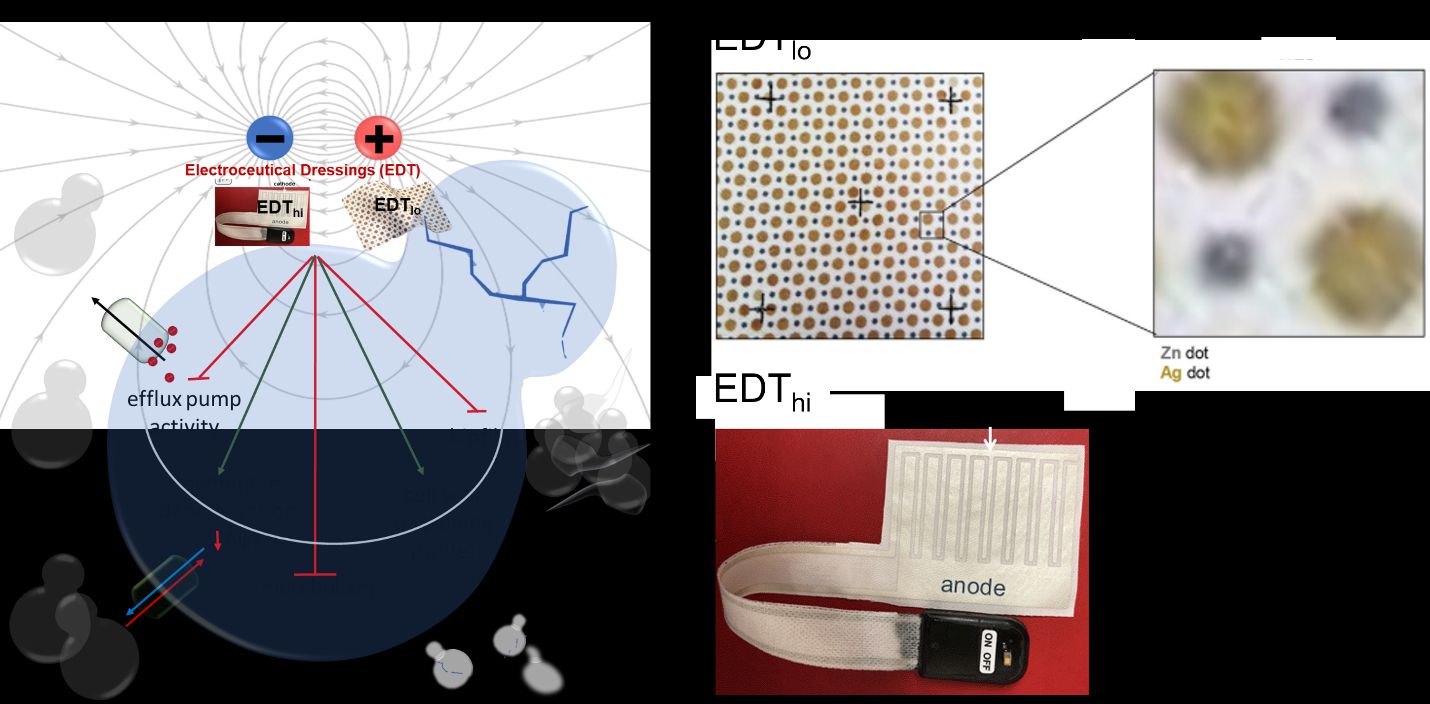
The EDTtechnologies comprises of EDTlo and EDThi dressings. The EDTlo or Wireless Electroceutical Dressing (WED,akaProcellera) is made of polyester fabric with printed alternating circular silver and zinc dots.The next-generation EDThi (aka PED-10), with inherent scalability of electric field for dosing, is an interdigitated pattern printed (medical grade silver) dressing powered by a 6V DC battery. “Electroceutical” refers to a low level of electricity provided to the wounds either via a matrix of embedded microcell batteries (silver and zinc dots) that wirelessly creates an electric field in the presence of moisture (e.g., sweat and wound exudate for EDTlo or via a 6V battery for EDThi wound dressing). These weak electric fields cause cell membrane depolarization and altered composition of the bacterial cell wall. Because of this, the application of EDTtechnology to a wound has the potential to make the bacteria more vulnerable to secondary stress.
Their product development cycle started with studies to determine the efficacy of EDTtechnology on the persistence of infections in burn wounds in a pig model. Burn wounds were infected with Pseudomonas aeruginosa andMethicillin-resistantStaphylococcus aureus(MRSA) and allowed to form biofilms over the wound. The team found that sequentialtreatment with EDT (first with EDThi followed by EDTlo) dressings significantly reduced infection, improved wound closure and reduced transepidermal water loss (TEWL), which is an indicator of proper functioning of skin in the wound healing process. On the cellular level, the team found that treatment with EDTtechnology significantly improved reepithelialization (resurfacing of new skin) and neovascularization (formation of new blood vessels) compared to the control group.
Following the success of this study, Dr. Roy’s team tested their EDTtechnology on the ketoconazole (an anti-fungal)-resistant yeast, Candida albicans. Findings indicated that EDTtechnology, either alone or in combination with ketoconazole, significantly reduced biofilm thickness and prevented hyphae formation (i.e., the long, tubular branching structures produced by fungi). This work was published in Bioelectrochemistry. This work was further validated in a pre-clinical porcine chronic wound model infected with Candida albicans. The EDTtechnology significantly improved wound healing similar to their results in the in vivo bacterial infection model.
This biophysical, as opposed to pharmacological, therapeutic principle may be leveraged to develop novel intervention strategies to prevent and manage bacterial and yeast infection.The team is now preparing a 510(k) for achieving FDA clearance of their EDThitechnology.EDTlo, on the other hand,has been FDA cleared (510(k) number K160783) and is available commercially as Procellera®).This project was funded through MTEC’s Request for Project Proposals for prototype acceleration (Solicitation #17-02-PA). The purpose of this effort was to help advance novel prototype technologies into the next major stage of development.
About the Roy Lab at Indiana University:
Dr. Sashwati Roy is a Professor of Surgery at the Indiana University School of Medicine and Director of Research at the IU Health Comprehensive Wound Center. She is an expert in inflammation, infection,and macrophage biology in chronic wounds. Dr. Sashwati Roy’s labs focuses on wound inflammation/infection, mechanisms of resolution of diabetic wound infection, tissue repair, and cellular plasticity.
Featured Member

Indiana University
Indiana University is dedicated to building a better future through education, research, and community engagement. With a commitment to academic excellence, IU offers a diverse range of programs across its nine campuses, fostering an inclusive environment for over 90,000 students from around the world. IU is also at the forefront of groundbreaking research, particularly in areas such as brain health and Alzheimer's disease.
Visit Profile