BurRapid Epidural Drainage Attachment for Traumatic Brain Injury
Published on April 03, 2025
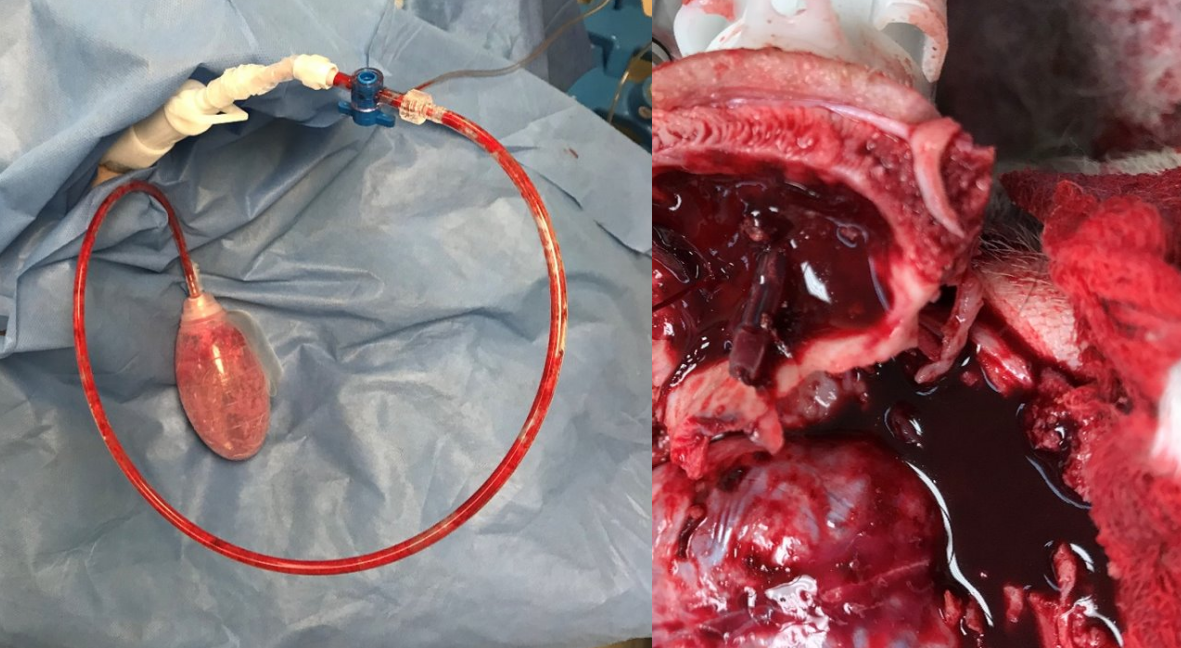
Epidural bleeding from traumatic brain injury (TBI) is immediately life-threatening and can quickly lead to brain herniation and death unless there is immediate neurosurgical intervention. BurRapid™, developed by Critical Innovations, is a medical device that provides hematoma drainage and intracranial pressure monitoring to sustain TBI patients on the battlefield. This allows for provision of these life-saving interventions earlier in the roles of care, including at the point-of-injury.
Project Highlight
With support from USAMRDC’s Combat Casualty Care Research Program (CCCRP), and MTEC, Critical Innovations, LLC is developing the BurRapidTM Intracranial Access Platform. The device, which will directly impact service members with traumatic brain injury (TBI), has undergone extensive military end-user testing and delivered very encouraging porcine study survival data.
Epidural bleeding from TBI is immediately life-threatening and can quickly lead to brain herniation and death unless there is emergent neurosurgical intervention. BurRapid™ is a mobile, low-weight, small-cube, low-power, modular, interoperable, ruggedized, temperature-stable, simplified, long shelf-life, and low-cost medical device that provides automated burr-hole access with subsequent hematoma drainage, ICP monitoring, and other neurosurgical procedures to sustain TBI patients on the battlefield. This allows for provision of these life-saving interventions earlier in the roles of care, including at the point-of-injury.
The BurRapid™ Intracranial Access Device allows a far-forward operator to easily establish intracranial access through an automated, non-surgical procedure with the device auto-sensing and auto-stopping once reaching the epidural space. This will help speed the time for an injured warfighter to receive definitive treatment while maximizing safety and minimizing complications.
So far, the program has met or exceeded all deliverables, been received with enthusiasm during extensive military end-user testing, and delivered very encouraging porcine study survival data. As demonstrated in a large animal model of epidural hemorrhage, use of the BurRapid™ Intracranial Access Platform dramatically reduced intracranial pressure (ICP) and prevented death in treated animals. The study was performed in live swine using a new epidural hemorrhage model developed specifically for this purpose, which included continuous active bleeding leading to brain death in about 45 minutes if left untreated. Use of the BurRapid™ Intracranial Access Platform successfully evacuated blood and dramatically reduced ICP, leading to survival after a full hour of continuous bleeding. Findings provide evidence that this innovative system will be able to stabilize TBI casualties by preventing and mitigating secondary brain injury from bleeding and increased ICP.Critical Innovations is currently transitioning the device towards Current Good Manufacturing Practice (cGMP) production and is advancing toward additional animal studies and a regulatory submission. See an overview of their medical devices here.
This project was funded through MTEC’s Request for Project Proposals for life saving trauma innovations (Solicitation #19-08-MuLTI).
The BurRapid™ technology is INVESTIGATIONAL and NOT AVAILABLE FOR COMMERCIAL SALE. These statements have NOT been evaluated by the Food and Drug Administration and this technology is NOT currently approved for human use.
Featured Member

Critical Innovations LLC
Critical Innovations LLC is a medical research and development company focused on creating cutting-edge solutions to health threats across pre-hospital, hospital, and military environments. The company focuses on task-shifting evidence-based interventions to earlier echelons of care and more general specialty levels, to simplify management and allow for rapid provision in emergency and combat environments. The company is dedicated to developing innovative medical devices and technologies that enhance emergency care and trauma management, particularly in military settings.
Visit Profile