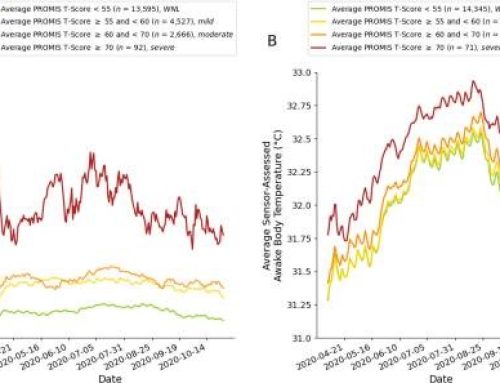
With support from the U.S. Army Medical Research & Development Command (USAMRDC) and MTEC, the Vanderbilt Center for Bone Biology (VCBB) is developing an injectable, settable bone void filler (BVF). Vanderbilt has demonstrated bone healing in preclinical models and developed commercial-scale manufacturing processes for their bone void filler technology, which has passed ISO 10993 biocompatibility tests. This project was funded through MTEC’s Request for Project Proposals for regenerative medicine manufacturing and prototyping (Solicitation #16-01-REGEN).
The design of biomaterials that mechanically stabilize fractures while remodeling to form new bone is an unmet challenge in bone tissue engineering. Open fracture injuries are one of the largest sources of morbidity on both the battlefield and in the civilian population. Over 80% of battlefield-injured warriors have at least one extremity wound, the majority of which are penetrating soft tissue injuries or open fractures. BVFs are synthetic formulations that are injected into a bone void caused by an injury. The BVF hardens and provides mechanical support as well as a scaffold for new bone growth. The overall goal is to achieve an optimal balance between bone growth rate and BVF resorption rate.
Specifically, the team at Vanderbilt has designed nanocrystalline hydroxyapatite (nHA)-poly(thioketal urethane) (PTKUR) bone cements that induce osteogenic differentiation of endogenous progenitor cells, exhibit bone-like mechanical properties, and undergo cell-mediated oxidative resorption. These differentiating factors of nHA-PTKUR BVFs and bone cements are anticipated to improve outcomes of open fractures in both military and civilian populations.

An example of the nanocrystalline hydroxyapatite (nHA)-poly(thioketal urethane) (PTKUR) bone cement. Using nHA-PTKUR, Scott Guelcher and his team at Vanderbilt have successfully demonstrated bone healing in preclinical models. These novel bone cements are expected to help warfighters recover from fracture injuries.
Vanderbilt’s nHA-PTKUR cements were implanted in femoral plug defects in rabbits and the formation of new bone and cartilage was evaluated between 4-18 months. As expected, intramembranous bone formation was observed near the periphery of the cement at 4 months. Additionally, endochondral bone growth was observed in the interior of the cement at 12 months. Endochondral bone growth of nHA-PTKUR in response to oxidative degradation by infiltrating chondrocytes (i.e. cartilage cells) is anticipated to maintain structural support while promoting remodeling at a rate aligned with patient biology. Recently, Vanderbilt developed Good Laboratory Practice (GLP) manufacturing processes and stable packaging configurations for nHA-PTKUR BVFs and bone cements, and their materials have passed the cytotoxicity, sensitization, intracutaneous reactivity, pyrogenicity, and acute systemic toxicity ISO 10993 biocompatibility tests. You can read more about their study published in ACS Biomaterials Science & Engineering. Future studies will include a pivotal preclinical study to demonstrate substantial equivalence to predicate BVFs and bone cements and GMP manufacturing development to support a 510(k) regulatory filing and clinical trial.
About the Vanderbilt Center for Bone Biology:
The Vanderbilt Center for Bone Biology (VCBB) was created to investigate diseases of bone and mineral metabolism. Investigators associated with the VCBB are engaged in basic, translational, and clinical research in musculoskeletal health. The goal of VCBB is to unravel novel biological mechanisms and to develop new treatments and diagnostic tools that can improve the quality of life for patients with bone destruction due to disease or trauma. The project is led by Scott Guelcher, PhD, a Professor of Chemical and Biomolecular Engineering and Director of the Vanderbilt Center for Bone Biology. Craig Duvall, PhD, the Cornelius Vanderbilt Professor of Biomedical Engineering, leads the development of the thioketal technology.